Interview with the CEO of RMeditech, a company specializing in lasers in the medical and beauty fields
31 certificates including An Visa and FDA… “Free from ‘needle fear’ with laser”
The final stage of product launch of blood collection device + blood sugar device… IPO challenge in 2 years
‘diabetes’. Because it is a disease that you have to live with for the rest of your life, there are many inconveniences in managing it. If the blood collection device is not disinfected regularly, it is easy to be exposed to secondary infections, and the ‘fear of a needle’ felt every time blood is drawn can lead to refusal to treat diabetes. For this reason, recently, companies that use bloodless blood glucose meters or ‘laser blood samples’ are attracting attention from diabetic patients.
Choi Jong-seok, CEO of Rameditech, who recently met with Dailyan, said, “The biggest difference from the needle point is that there is no needle.” can,” he introduced.
He said, “Needle bleeders physically tear the skin, so there is a fatal disadvantage of infection or calluses.” “The laser lancet draws only the necessary peripheral blood through energy instead of a needle, so there are fewer wounds, and secondary There is no worry regarding infection,” he said.
Rameditec is a laser specialized company in the medical and beauty field, established in January 2012 by researchers from Samsung Advanced Institute of Technology. Its main products include ‘Handy Ray’, a laser blood sampling device that can be used for inspection of diabetic patients and on-site diagnostic devices, ‘Puracell’ used for treatment and beauty, and ‘Ravet’, a treatment device for companion animals.
The strength of Rameditech’s products is ‘safety’, which has been recognized at home and abroad. In particular, it was certified by ANVISA of Brazil, which is difficult to obtain. It has a total of 31 certifications and patents, from KGMP, a domestic standard that conforms to the international standard of the medical device quality management system (ISO13485), to the US Food and Drug Administration (FDA) certification. CEO Choi explained, “It is much less painful than the needle sampling machine, and the amount of blood is the same.” “I was concerned regarding blood denaturation due to the strong heat as I used the laser, but the blood glucose values of the blood sampled from the needle sampling machine were the same.”
개발에서 판매까지 8년…중국 업체 유혹도 뿌리친 '고집'
 Handy Ray Light (left) and Handy Ray Pro ⓒ Rameditec
Handy Ray Light (left) and Handy Ray Pro ⓒ RameditecAlthough it is unfamiliar, laser blood sampling machines have already existed. There were several laser blood collection equipment developers in Korea, including the United States and China. However, their products were too heavy and failed to commercialize due to the disadvantage of not being able to draw blood, which is a key function. In addition, the price difference with the needle blood sampling machine, which can be said to be a competitive product, was a whopping 20 times.
CEO Choi started developing a laser blood collection device called ‘Handy Ray’ by supplementing the shortcomings of these existing products. However, this process was not smooth. It took three years to develop a laser blood sampling device, three years to obtain medical device approval from the Ministry of Food and Drug Safety, and two years to secure clinical data for hospital sales. It took a total of 8 years from development to sales. Instead of making money during this period, there was only a deficit.
CEO Choi Jong-seok said, “It took 3 years to increase the weight and usability by removing unnecessary optical parts in large laser equipment. When I finished selling it to hospitals, they asked for clinical data. Researchers gathered and developed only, so I didn’t know much regarding the medical market. It took a total of 8 years from product development to sale.”
Since the sales for 8 years were ‘0’ won, there was also a temptation to sell the company’s technology. He said, “Some companies that highly evaluated the company’s technology or Chinese companies offered to sell the company, but they didn’t sell it because of the researcher’s pride or stubbornness.”
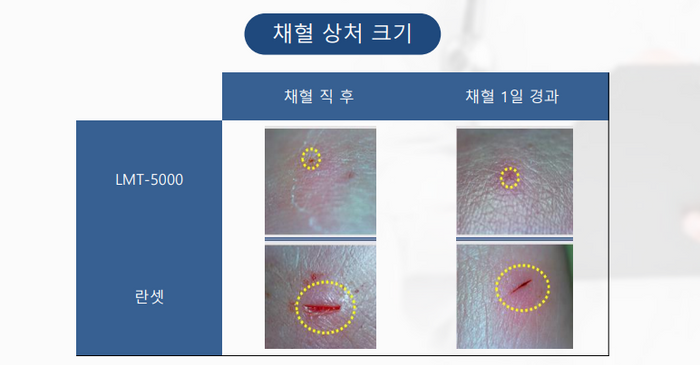 Comparison of wound sizes between Rameditech’s laser blood sampler (top) and needle sampler ⓒRameditech
Comparison of wound sizes between Rameditech’s laser blood sampler (top) and needle sampler ⓒRameditechThe response of the laser blood collection device, which came out following 8 years, was successful. In particular, it received a great response from parents with newborns or premature babies. This was because the size of the blood collection wound was small and the pain of blood collection for children was dramatically reduced. CEO Choi said, “Handy Ray is evaluated to be less painful than the products that are rumored to be painless. Babies tear their soles of the feet to collect blood. What do parents think of this? Parents raising children show a positive reaction.” said
However, it has not yet reached the stage of dissemination. This is because the price is still higher than that of a needle lancet and it is not covered by insurance. The Health Insurance Corporation is hoping for government support, such as the ‘consumable material purchase cost support system’ provided to diabetic patients. He said, “Even though the cap (disposable) of the Handyray product is a consumable material, it is not covered by insurance.” “If insurance is applied, the one-time usage fee will drop by 10 won. The price will come down.”
내년 목표 매출은 150억원이상…IPO도 준비 중
CEO Choi Jong-seok’s goal is to go public. RMeditech’s sales last year were 1 billion won. This year’s goal is 5 billion won, and next year’s target is 15 billion won or more. “We are preparing for an IPO in the second half of 2023 or the first half of 2024,” he said. Rameditec plans to introduce a personal beauty device in the second half of this year, and a product that combines a blood draw and blood glucose device in the first half of next year.
CEO Choi explained, “Development is already over. We are waiting for approval from the Ministry of Food and Drug Safety.” He explained, “It is a product that can be linked with mobile devices for health management.” He continued, “Recently, we have completed a contract with a global distribution company. We are planning to release it in Korea within this year, and we are planning to sell it all over the world next year,” he said.
In the future, we plan to develop a ‘needleless syringe’ by utilizing our laser miniaturization technology. The goal is to release a product that can be used as a drug for dementia, arthritis, or atopic dermatitis. Currently, animal clinical trials are underway with insulin and ischemic lower extremity necrosis drugs.
©Dailyan Co., Ltd. Unauthorized reproduction and redistribution prohibited