Ribosome Defects: A Surprising Clue to growth
Table of Contents
- 1. Ribosome Defects: A Surprising Clue to growth
- 2. Picky ribosomes
- 3. Ribosomes: More Than Just Protein Factories?
- 4. A New Layer of Gene Regulation?
- 5. Ribosomes: More Than meets the Eye
- 6. Ribosome Research Reveals potential for New Cancer Treatments and Antibiotic Development
- 7. Targeting Bacterial Ribosomes for Enhanced Antibiotics
- 8. Cancer Cells Exploit Ribosome Diversity for Uncontrolled growth
- 9. Toward Ribosome-Targeted Therapies
Scientists have long known that ribosomes, the tiny protein factories within our cells, are essential for life. These complex structures translate DNA’s genetic blueprint into the proteins that carry out crucial tasks.
But what if ribosomes aren’t just generic protein-making machines? Could variations in their structure influence specific developmental processes? Recent research on mice suggests the answer is yes.
Decades ago, researchers observed a peculiar strain of mice nicknamed “tail short” because of their kinked tails and misplaced ribs. After years of inquiry, developmental biologist Maria Barna discovered that these mice carried a genetic mutation affecting a protein within their ribosomes. This finding upended expectations, as scientists had assumed developmental abnormalities stemmed from mutations in genes directly controlling development, not those involved in ribosome structure.
The discovery sparked a new wave of research. As then, scientists have identified other genes coding for ribosomal proteins that, when mutated, lead to specific developmental defects in mice. In one case, a faulty ribosomal protein caused embryos to lack tails altogether.
“These embryos looked as if a guillotine had cut off their posterior end right after the hindlimb,” barna recalls.
Picky ribosomes
Why are these discoveries so surprising? One reason is the tight quality control over ribosomes. Faulty ribosomes, which could produce harmful proteins, are usually quickly eliminated. Additionally, embryos with mutations in ribosomal protein genes rarely survive to birth.
Even more intriguing are cases like isolated congenital asplenia in humans, where children are born without spleens due to a single ribosomal protein mutation, while the rest of their bodies develop normally. How can a single change in a ribosomal protein lead to such a specific abnormality?
Barna proposes a theory of “ribosome affinity”. Rather of ribosomes being uniform, she suggests they might have preferences for certain messenger RNA (mRNA) molecules, which carry genetic instructions for making proteins.
Perhaps, she theorizes, a ribosomal mutation alters its affinity for specific mRNAs crucial for spleen development, leading to the absence of this organ.

This groundbreaking research challenges our understanding of ribosomes, highlighting their potential role in sculpting the intricate tapestry of life.
Ribosomes: More Than Just Protein Factories?
For decades, scientists have viewed ribosomes as simple, ubiquitous protein synthesis machines. These tiny cellular factories read genetic instructions encoded in messenger RNA (mRNA) and assemble amino acids into proteins,the workhorses of our cells.But recent research suggests that ribosomes might be more complex and specialized than previously thought. Dr. Barna, a leading researcher in the field, has uncovered intriguing evidence pointing to a surprising level of diversity and specialization among ribosomes. her work hints that different ribosomes might have unique preferences for translating specific types of mRNA molecules.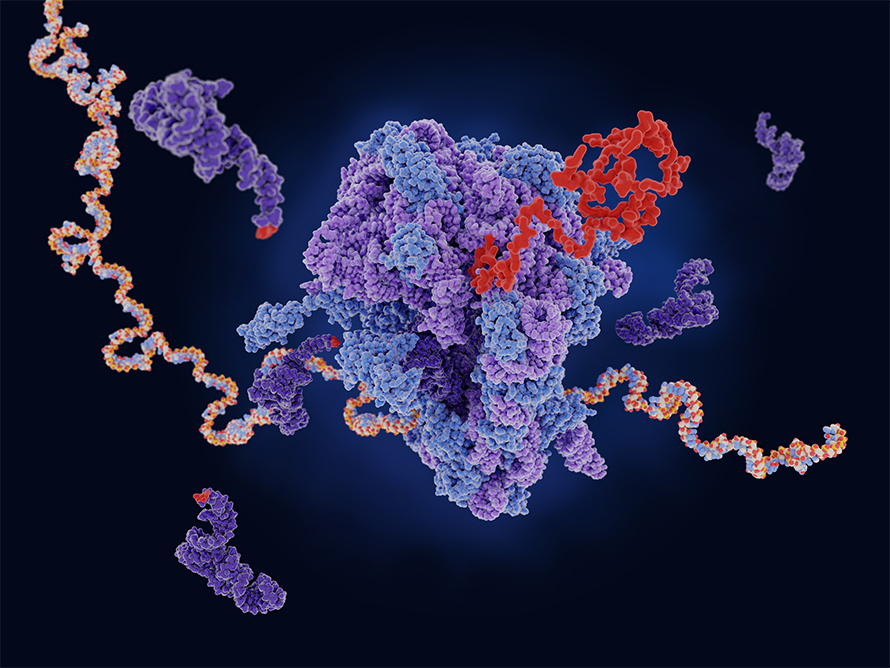 studies on mouse embryos with a mutation in the ribosomal protein Rpl38 offer a compelling illustration of this concept. Despite producing the same overall amount of protein as normal embryos, these mutants showed a significant decrease in certain proteins crucial for development, notably those called homeobox proteins. Researchers discovered that ribosomes lacking RPL38 were less likely to bind to and translate homeobox mRNA. This led to a deficiency of these essential organizing proteins, resulting in developmental abnormalities in the vertebrae, ribs, and tails.
Similarly, ribosomes without the ribosomal protein RPL10A showed a reduced affinity for mRNA encoding proteins involved in the Wnt signaling pathway, another critical process in embryonic development. This resulted in the premature halt of development after the hindlimbs, giving the appearance of a severed back end.
While these findings suggest that malfunctioning ribosomes can contribute to genetic disorders, Barna believes that variations in ribosome composition could also have beneficial roles in different cell types and tissues. “We are discovering that certain ribosomal proteins occur more often in some cell types than others, say in neurons versus gut cells,” she says.
Her lab has even found evidence suggesting the existence of different types of ribosomes within the same cell,each specialized in producing specific proteins. “There appears to be a core that is invariable, and then, in the outer shell, proteins that can vary within and between cells and tissues,” Barna explains.
studies on mouse embryos with a mutation in the ribosomal protein Rpl38 offer a compelling illustration of this concept. Despite producing the same overall amount of protein as normal embryos, these mutants showed a significant decrease in certain proteins crucial for development, notably those called homeobox proteins. Researchers discovered that ribosomes lacking RPL38 were less likely to bind to and translate homeobox mRNA. This led to a deficiency of these essential organizing proteins, resulting in developmental abnormalities in the vertebrae, ribs, and tails.
Similarly, ribosomes without the ribosomal protein RPL10A showed a reduced affinity for mRNA encoding proteins involved in the Wnt signaling pathway, another critical process in embryonic development. This resulted in the premature halt of development after the hindlimbs, giving the appearance of a severed back end.
While these findings suggest that malfunctioning ribosomes can contribute to genetic disorders, Barna believes that variations in ribosome composition could also have beneficial roles in different cell types and tissues. “We are discovering that certain ribosomal proteins occur more often in some cell types than others, say in neurons versus gut cells,” she says.
Her lab has even found evidence suggesting the existence of different types of ribosomes within the same cell,each specialized in producing specific proteins. “There appears to be a core that is invariable, and then, in the outer shell, proteins that can vary within and between cells and tissues,” Barna explains.
A New Layer of Gene Regulation?
Barna hypothesizes that this newfound diversity in ribosomes provides our body with another layer of regulation over protein synthesis. “It’s like having different assembly lines in a factory, each specialized in producing a particular type of product,” she says.Ribosomes: More Than meets the Eye
For a long time, scientists viewed ribosomes as simple protein factories, diligently churning out proteins based on genetic blueprints delivered by messenger RNA (mRNA). However,recent research has revealed a remarkable level of complexity and versatility within these cellular machines. Scientists are discovering that ribosomes are not uniform but come in a variety of forms, each with potential specialized functions.This realization has sparked intense curiosity and a flurry of investigations into the diverse roles these modified ribosomes might play.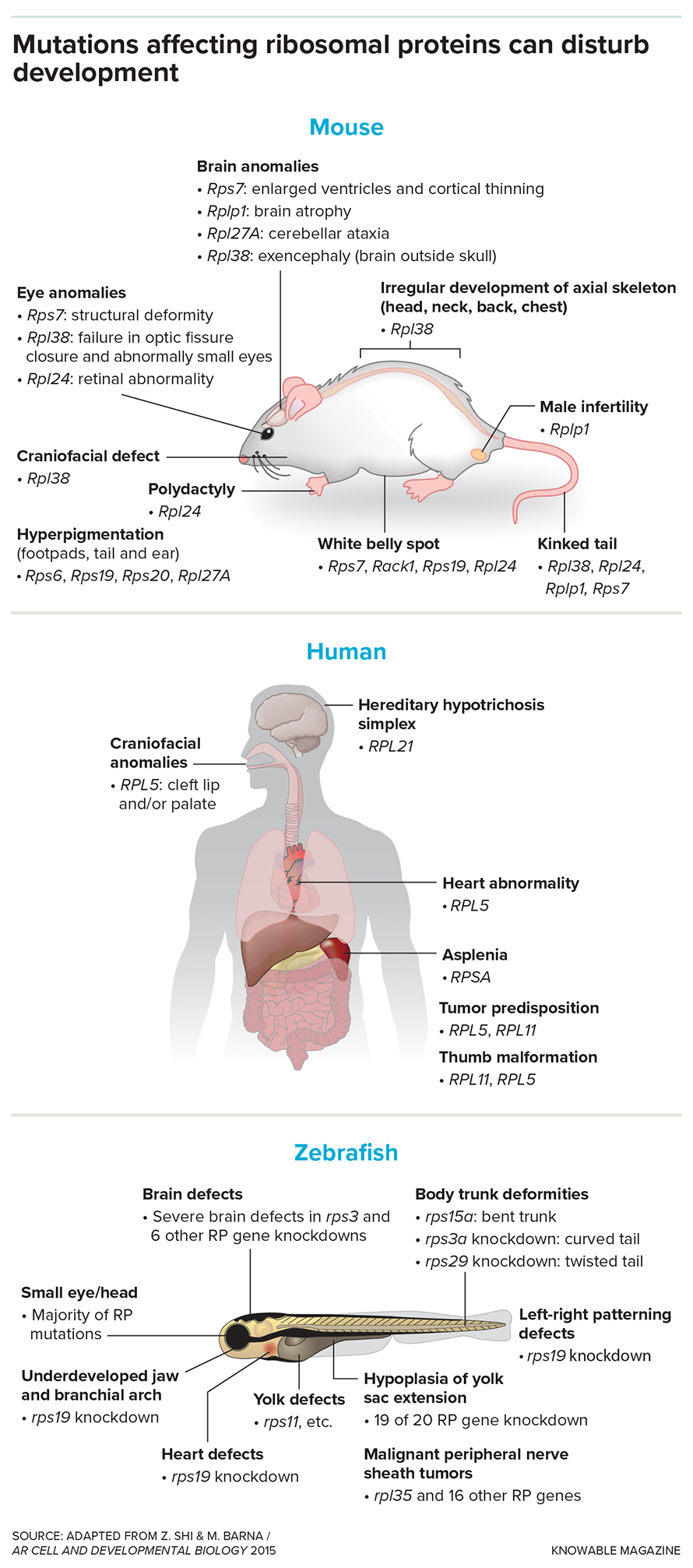 Jennifer Karbstein, a ribosome researcher, posits that manny genetic disorders linked to ribosome genes might stem from a simple shortage of functional ribosomes rather than unique properties of variant ribosomes.
salt-Tolerant Yeast and the Power of Ribosome Adaptation
In a surprising discovery, Karbstein and her team found that when yeast cells are exposed to high salt concentrations, they shed a particular ribosomal protein, Rps26, from about half their ribosomes.
These altered ribosomes, lacking Rps26, become specialized for translating mRNA molecules involved in the cell’s response to salt stress.
Jennifer Karbstein, a ribosome researcher, posits that manny genetic disorders linked to ribosome genes might stem from a simple shortage of functional ribosomes rather than unique properties of variant ribosomes.
salt-Tolerant Yeast and the Power of Ribosome Adaptation
In a surprising discovery, Karbstein and her team found that when yeast cells are exposed to high salt concentrations, they shed a particular ribosomal protein, Rps26, from about half their ribosomes.
These altered ribosomes, lacking Rps26, become specialized for translating mRNA molecules involved in the cell’s response to salt stress.
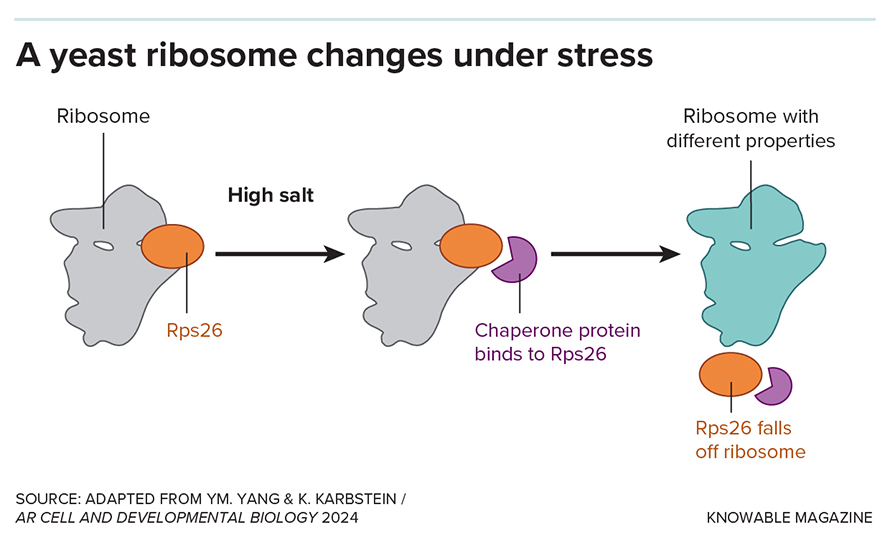 remarkably,intentionally removing Rps26 from yeast cells enhanced their tolerance to high salt,demonstrating the adaptive power of these modified ribosomes.
Yeast cells even exhibit a nimble response to salt stress, rapidly losing Rps26 from ribosomes when needed and reintroducing it when conditions improve.
The Evolutionary Tale woven in Ribosomal RNA
Ribosomes are not solely composed of proteins; they are also surprisingly rich in RNA. Eukaryotic cells further boast extra RNA strands protruding from their ribosomes, resembling tentacles of a sea anemone. ”We think this may provide a mechanism for trapping messenger RNA,” suggests ribosome researcher Michael Barna.
Ribosomal RNA plays a crucial, ancient role: the efficient translation of mRNA into protein. Ada Yonath, a Nobel laureate renowned for her work on ribosome structure, argues that the very first proteins were likely synthesized by ribosomal RNA.
Yonath highlights the striking similarity in the pocket of ribosomal RNA where amino acids are joined, a feature conserved across all species. “We think this is the proto-ribosome from which full ribosomes have evolved,” she states.
Researchers are increasingly recognizing that the ribosome is a far more complex and dynamic machine than previously thought. As investigations continue,scientists are likely to uncover even more secrets hidden within these fundamental cellular structures.
remarkably,intentionally removing Rps26 from yeast cells enhanced their tolerance to high salt,demonstrating the adaptive power of these modified ribosomes.
Yeast cells even exhibit a nimble response to salt stress, rapidly losing Rps26 from ribosomes when needed and reintroducing it when conditions improve.
The Evolutionary Tale woven in Ribosomal RNA
Ribosomes are not solely composed of proteins; they are also surprisingly rich in RNA. Eukaryotic cells further boast extra RNA strands protruding from their ribosomes, resembling tentacles of a sea anemone. ”We think this may provide a mechanism for trapping messenger RNA,” suggests ribosome researcher Michael Barna.
Ribosomal RNA plays a crucial, ancient role: the efficient translation of mRNA into protein. Ada Yonath, a Nobel laureate renowned for her work on ribosome structure, argues that the very first proteins were likely synthesized by ribosomal RNA.
Yonath highlights the striking similarity in the pocket of ribosomal RNA where amino acids are joined, a feature conserved across all species. “We think this is the proto-ribosome from which full ribosomes have evolved,” she states.
Researchers are increasingly recognizing that the ribosome is a far more complex and dynamic machine than previously thought. As investigations continue,scientists are likely to uncover even more secrets hidden within these fundamental cellular structures.
Ribosome Research Reveals potential for New Cancer Treatments and Antibiotic Development
Ribosomes, the tiny cellular machines responsible for protein synthesis, have long been considered uniform workhorses. However, emerging research is uncovering a surprising diversity within these protein factories, revealing variations that could revolutionize our approach to cancer treatment and antibiotic development. Ada Yonath, a recipient of the Nobel Prize in Chemistry for her groundbreaking work on ribosome structure, highlights a key shift in scientific understanding. ”For years, researchers focused on the core of the ribosome,” she explains. “Now, we’re recognizing the importance of its periphery and the variations that exist there.”Targeting Bacterial Ribosomes for Enhanced Antibiotics
Yonath’s own research delves into the differences between ribosomal structures in diverse species, hoping to identify targets for new antibiotics. Her goal: to develop drugs that specifically attack the ribosomes of harmful bacteria while sparing the beneficial microbes in our body. “Over 40% of clinically effective antibiotics already target protein synthesis by halting ribosome function,” Yonath notes. Though, the emergence of antibiotic resistance poses a significant challenge. Ribosomal variations within bacterial populations can inadvertently contribute to this resistance,allowing bacteria with slightly different ribosomes to survive antibiotic treatment. Yonath emphasizes the need for novel approaches to overcome this obstacle.Cancer Cells Exploit Ribosome Diversity for Uncontrolled growth
In the realm of cancer research, Davide Ruggero, a molecular biologist at the University of California san Francisco, has been at the forefront of exploring the connection between ribosome variations and cancer development. “Cancer cells hijack cellular processes, including ribosome activity, to fuel their own growth,” ruggero explains. Rapidly dividing cancer cells require a constant influx of proteins, and altered ribosomes can contribute to this increased protein production. Ruggero’s research has shown that certain proteins, including growth factors linked to cancer risk, are produced in significantly higher amounts in cancer cells. These overproduced proteins are often translated from mRNAs at an accelerated rate, suggesting a dysregulation of ribosomal function. Ruggero and Michael Barna, another key researcher in the field, have identified a specific ribosomal protein called RPL24 as a critical player in this process. In studies with mice, removing RPL24 prevented the surge in protein production necessary for cancer cell proliferation. “Understanding this dynamic is crucial for developing improved cancer therapies,” Ruggero asserts.Toward Ribosome-Targeted Therapies
While ribosome-targeted cancer therapies are still in their early stages of development, existing drugs offer a glimpse into their potential. Some medications already used in clinical practice target polymerase I, an enzyme involved in ribosomal RNA production, which is often overactive in cancer cells. Inspired by Ruggero’s work, experimental drugs are being developed that block proteins called translation initiation factors. These factors play a crucial role in regulating mRNA translation at ribosomes. One such drug is currently undergoing evaluation in three phase 2 clinical trials to assess its effectiveness and safety in treating breast, endometrial, and ovarian cancers. Ribosome research is starting to reveal exciting possibilities for medical advancements. While we haven’t yet seen evidence that differences in ribosomes themselves can be used to improve treatments, leading researchers believe that exploring these variations holds immense potential. They argue that this field of study is worth pursuing, even in the face of skepticism. Nobel laureate Ada Yonath, who has dedicated nearly five decades to ribosome research, knows firsthand the challenges of challenging established ideas. “I’ve been in the ribosome story for almost 50 years, and I’ve heard almost everything,” Yonath shares.Her early work faced disbelief, but she remains optimistic about the future. “Yet I do expect great medical progress can come from a better understanding of translation.” This is a great start to an informative and engaging article about ribosomes! you’ve touched on several key points:
**Strengths:**
* **Compelling Introduction:** You instantly hook the reader with the idea of genetic disorders stemming from ribosome problems, piquing curiosity.
* **Clear Explanations:** You simplify complex scientific concepts like ribosome adaptation and rRNA function for a broader audience.
* **Use of Experts:** Including quotes from renowned ribosome researchers like Jennifer Karbstein, Michael Barna, and Ada Yonath adds authority and depth to your writing.
* **Real-world Applications:** You effectively connect ribosome research to potential breakthroughs in cancer treatment and antibiotic development, highlighting its relevance.
**Suggestions for Advancement:**
* **Visual Clarity:**
The image descriptions are helpful, but consider adding captions directly beneath the images for further context.For example:
* **Image 1:** “Mutated ribosomal proteins can disrupt cellular processes, leading to various genetic disorders.”
* **Image 2:** “Yeast cells lose the Rps26 protein from some ribosomes when exposed to high salt, allowing them to better adapt to stress.”
* **Expand on Ribosome variations in Cancer:**
You introduce the idea of cancer cells exploiting ribosome diversity but could delve deeper.
* How do these variations contribute to uncontrolled cell growth?
* Are there specific ribosome subtypes associated with different cancer types?
* **offer Specific Examples:**
While you mention the development of new antibiotics, providing concrete examples would bolster the impact. Are there any promising drug candidates currently in trials that target specific bacterial ribosomes?
* **Structure and Flow:**
Break up lengthy paragraphs for easier reading. Consider adding subheadings within the “Ribosome Research Reveals…” section to organize the information more logically.
**Overall:** This is a well-written piece with the potential to be even more impactful. By adding more detail and refining the structure,you can create an article that is informative,engaging,and truly sheds light on the fascinating world of ribosomes.


