2024-07-12 06:00:04
The quest for green hydrogen, essential for a clean energy transition, has just reached a milestone thanks to a major scientific breakthrough. A European team, led by the Institute of Photonic Sciences (ICFO) of Barcelonahas developed a new catalyst for’electrolysis water, paving the way for industrial production ofhydrogen without recourse to materials rare and expensive like iridium.
Hydrogen is a promising energy vector to decarbonize our society. Unlike fossil fuels, its use as a fuel does not generate carbon dioxide. However, the majority of hydrogen produced today comes from a process that generates significant CO2 emissions. To obtain green hydrogen, alternatives more sustainable ones are needed.
Water electrolysis is a potential method to produce green hydrogen using renewable energy. This process relies on catalysts at the anode and cathode to accelerate the splitting of water into hydrogen and oxygen. Among the electrolysis technologies, the proton exchange membrane (PEM) is particularly promising due to its high energy efficiency and high production rates.
Traditionally, water electrolysis, particularly PEM technology, requires catalysts based on rare elements such as platinum and iridium. These materials, although efficient, pose cost and availability problems, especially iridium, one of the rarest elements on Earth. Finding alternatives to these materials is therefore a major challenge.
The ICFO team addressed this challenge by developing a new catalyst based on cobalt, an abundant and economical metal. Their innovative approach relies on using the properties of water to stabilize the catalyst. By integrating water and water fragments into the catalyst structure, they were able to improve its stability in acidic environments, typical of PEM electrolyzers.
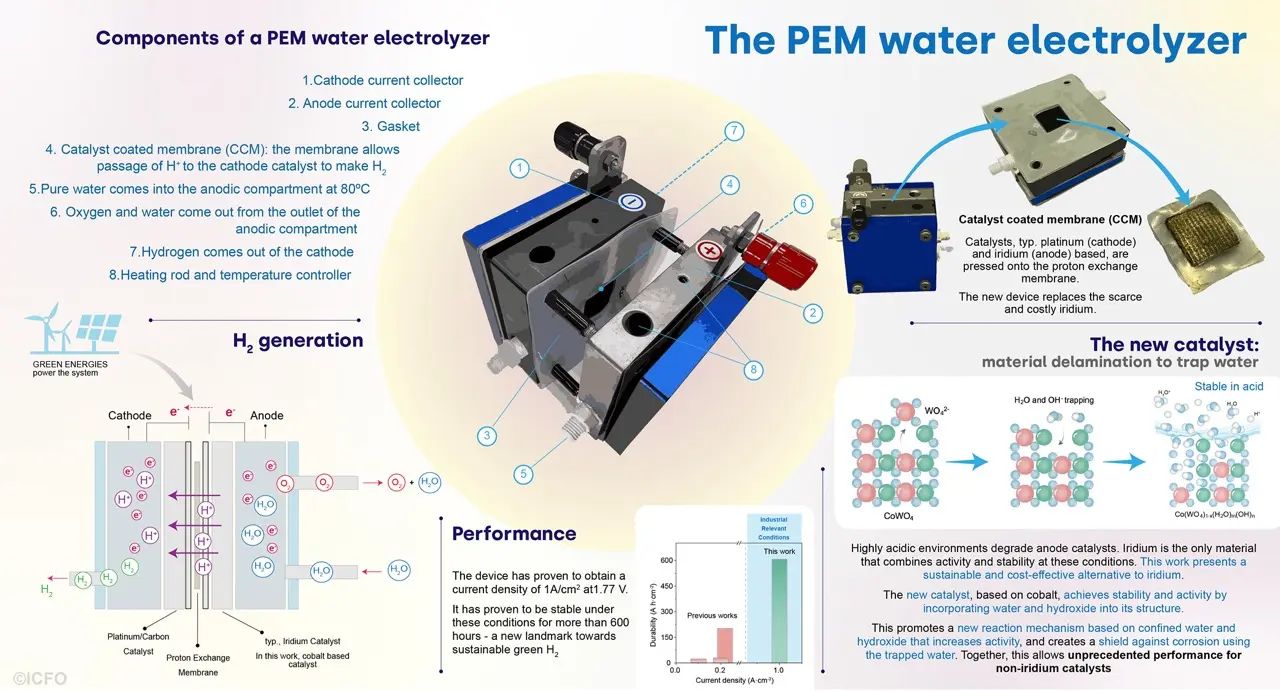
This breakthrough was made possible by a process called delamination. By treating a cobalt-tungsten oxide with basic aqueous solutions, the researchers were able to replace the tungsten oxides with water molecules and hydroxyl groups. This new material showed remarkable performance in terms of stability and activity under high-temperature industrial conditions. current density.
The results of this research were published in Sciencehighlighting a collaboration between ICFO and several prestigious institutions, including the Institute of Chemical Research of Catalonia (ICIQ), the Catalan Institute of Nanosciences and Nanotechnologies (ICN2), the National Center for Scientific Research (CNRS), Diamond Light Source and the Institute of Advanced Materials (INAM).
Dr. Lu Xia, co-lead author of the study, emphasizes the importance of this discovery: “We have achieved a current density of 1 A/cm², a significant milestone, with more than 600 hours of stability at this density. This is a major breakthrough for iridium-free catalysts.”
While this discovery represents an important step towards the industrialization of green hydrogen production, challenges remain, including catalyst lifetime and materials optimization. The team is already working on nickel and manganese-based alternatives to further improve performance and sustainability.
By filing a patent for this new technology, the researchers hope to accelerate its industrial adoption, thus contributing to the decarbonization of our society and the transition to cleaner renewable energies.
1720887340
#production #green #hydrogen #iridium #catalyst
