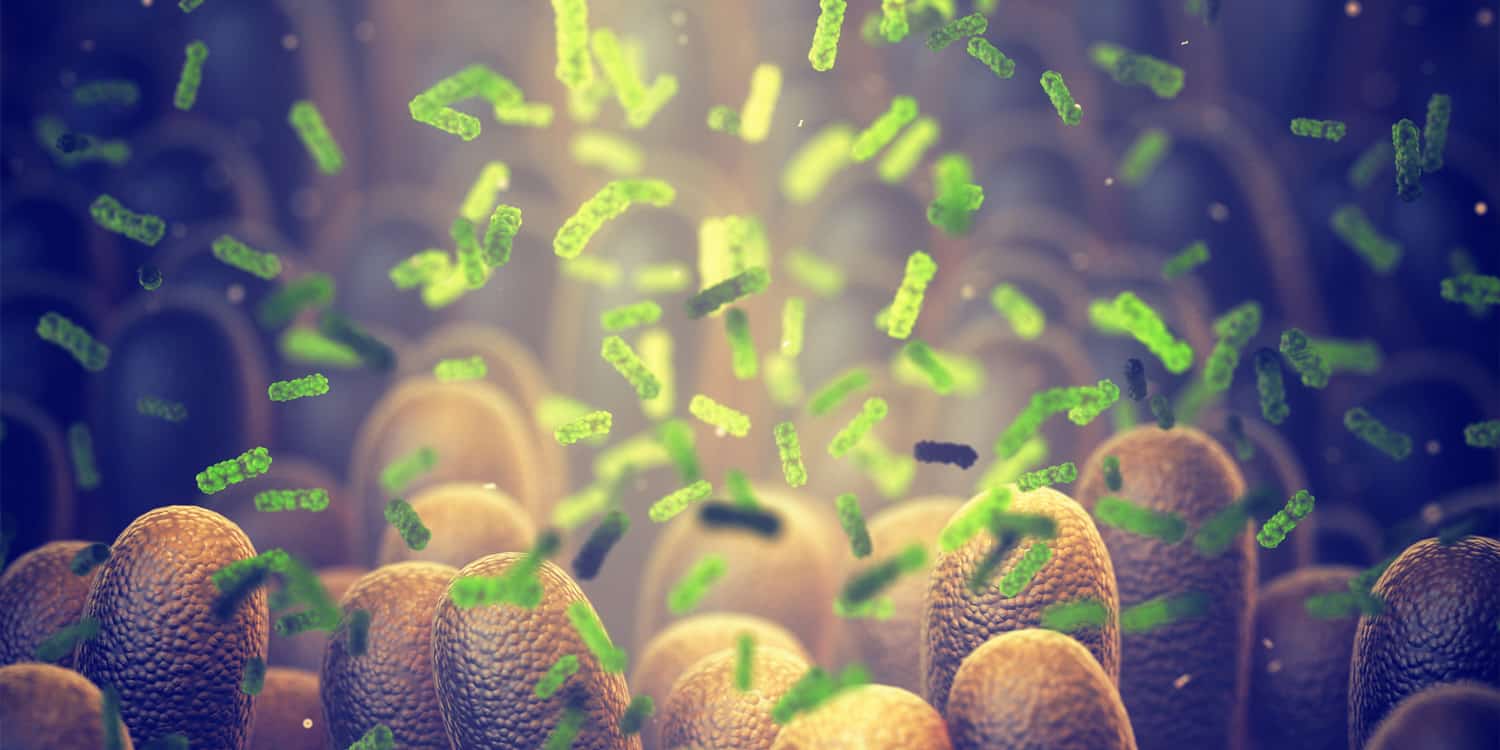
In a groundbreaking new study, researchers have utilized a sophisticated systems biology approach to investigate how gut bacteria metabolites may impact Alzheimer’s disease. This study, published in Cell Reports, combines artificial intelligence (AI), genetics, and multi-omics analyses to uncover novel insights into the development and potential treatment of this neurodegenerative disorder.
Alzheimer’s disease is a progressive condition that primarily affects older adults, leading to a decline in cognitive functions such as memory and reasoning. The underlying causes of this disease remain largely unknown, but it is believed to involve a combination of genetic, lifestyle, and environmental factors that affect the brain over time. Accumulation of amyloid-beta plaques and tau protein tangles in the brain interfere with neural function and result in cell death, further exacerbating the symptoms.
Prior research has indicated a correlation between changes in gut bacteria and the progression of Alzheimer’s disease. It has been observed that these bacteria produce metabolites, which can potentially influence brain health and contribute to the development of the disease. However, the specific pathways through which these metabolites act have remained a mystery.
This knowledge gap spurred the current study, which aimed to uncover the interactions between these metabolites and human receptors. Led by Feixiong Cheng and a team of experts from institutions including the Cleveland Clinic Genome Center and the Luo Ruvo Center for Brain Health, researchers employed machine learning algorithms to analyze over a million potential metabolite-receptor pairs. Genetic data and experimental validation using neurons derived from Alzheimer’s patients complemented these predictions and refined the understanding of the gut-brain axis in relation to Alzheimer’s disease.
One of the most significant findings of this study was the identification of specific G-protein-coupled receptors (GPCRs) that interact with metabolites produced by gut bacteria. The researchers focused on orphan GPCRs, which are receptors with unknown natural activators, and discovered that certain metabolites can activate these receptors. This discovery is particularly exciting, as it opens up new possibilities for drug development, targeting these receptors to potentially prevent or mitigate the progression of Alzheimer’s disease.
Among the metabolites investigated in the study, phenethylamine and agmatine stood out due to their effects on tau proteins. Tau proteins play a crucial role in the neurological degradation characteristic of Alzheimer’s disease, and the study revealed that these metabolites might significantly alter the levels of phosphorylated tau proteins in neurons derived from Alzheimer’s patients. Notably, agmatine demonstrated a protective effect by reducing harmful tau phosphorylation, making it a potential candidate for therapeutic development.
The application of machine learning models played a pivotal role in predicting and uncovering the interactions between over a million metabolite-receptor pairs. This high-throughput approach streamlined the identification of relevant interactions and facilitated a deeper understanding of the complex mechanisms through which gut microbiota can influence brain health. By integrating genetic analyses and experimental data, the researchers were able to validate their predictions and refine their understanding of the gut-brain axis in the context of Alzheimer’s disease.
While the study presents promising findings, the authors acknowledge several limitations. The complexity of the gut-brain axis necessitates further validation through experimental and clinical studies. Additionally, it is important to consider the broader physiological and environmental factors that may influence these processes in a living system.
Despite these limitations, this research provides a valuable framework for comprehending how metabolites from gut bacteria can impact brain health and disease. The implications of these findings extend beyond Alzheimer’s disease, as the methodologies and insights gained might potentially be applied to other neurological and systemic diseases influenced by the gut microbiota.
The results of this study have the potential to revolutionize the way we approach the treatment of Alzheimer’s disease and other conditions associated with gut microbiota. By targeting specific receptors and metabolites, researchers may be able to develop innovative therapeutic interventions that halt or slow down the progression of these diseases. The integration of AI, genetics, and multi-omics analyses has proven to be a powerful tool, enabling scientists to unravel the intricate connections between gut bacteria metabolites and human health.
This research aligns with current trends in precision medicine and personalized approaches to healthcare. As we continue to uncover the complex interplay between our microbiota, genetics, and disease, tailored therapies might become a reality. The potential to modulate specific receptors and metabolites opens up new avenues for drug development in various disease domains, not limited to Alzheimer’s disease.
Looking ahead, further exploration of the gut-brain axis and the role of metabolites might unlock groundbreaking treatments for a multitude of diseases. By harnessing the power of AI and combining it with experimental validation and clinical studies, we can accelerate the translation of these findings into practical applications. Future research should focus on confirming these interactions in living organisms and investigating the therapeutic potential of targeting these pathways in clinical settings.
The implications of understanding the gut-brain axis extend beyond neurological diseases and might impact a wide range of conditions influenced by the gut microbiota. By expanding our knowledge in this area, we pave the way for novel interventions and preventive strategies for a myriad of diseases.
This study acts as a stepping stone toward a future where we can leverage our understanding of gut bacteria metabolites to optimize human health. The potential is vast, and the discoveries made in this field have the capacity to transform healthcare and improve the lives of millions. As we continue to unravel the complexities of the gut-brain axis, we must be open to new possibilities and collaborative research endeavors that pave the way for a brighter and healthier future.