2024-03-18 00:52:35
Seoul National University College of Medicine Professor Muk In-hee, Seoul National University Dementia Convergence Research Center postdoctoral researcher Kim Dong-gyu, and postdoctoral researcher Choi Hyun-jeong (from the left in the photo)
[메디칼업저버 박선재 기자] Professor Mook In-hee’s research team at Seoul National University was the first to identify the interrelationship between Alzheimer’s disease pathology and hypothyroidism, especially thyroid hormone deficiency in the brain.
Thyroid hormones are important hormones for brain development and function, and imbalances in thyroid hormone levels are known to cause problems with brain health and are a risk factor for the onset and progression of Alzheimer’s disease, which causes cognitive impairment.
In particular, hypothyroidism shows significant similarities to symptoms of Alzheimer’s disease, such as decreased cognitive memory ability and brain fog.
Several epidemiological studies have reported changes in thyroid hormone levels in the blood, cerebrospinal fluid, and postmortem brain tissue of Alzheimer’s disease patients, but the clear mechanisms by which changes in thyroid hormone metabolic processes in the brain and thyroid hormone deficiency cause pathological changes are not yet known. .
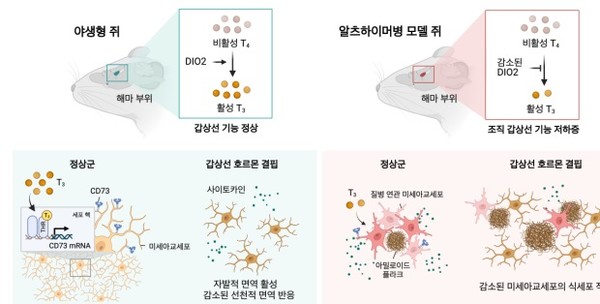
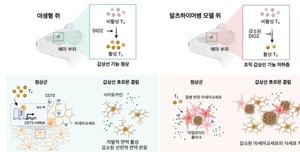
painting. In the brain of Alzheimer’s dementia mice, a decrease in thyroid hormone occurs due to a disorder in thyroid hormone metabolism caused by a lesion. In Alzheimer’s dementia mice, thyroid hormone deficiency reduces the phagocytosis of microglia, leading to the accumulation of beta-amyloid and phosphorylated tau and worsening cognitive and behavioral disorders.
The research team confirmed changes in thyroid hormone levels in the brains of mice with Alzheimer’s dementia and found that thyroid hormone levels in the hippocampus decreased from the early stages of the disease, and that this occurred faster than the decrease in blood thyroid hormone levels.
Through single cell analysis using brain tissue, the research team revealed that thyroid hormone deficiency has a significant impact on the function of microglia, immune cells residing in the brain.
Based on these research results, T3, an active thyroid hormone, was administered to treat Alzheimer’s dementia mice lacking thyroid hormone in the brain.
Thyroid hormones inhibit beta-amyloid in the brain.
It was discovered that microglial cells play a role in forming immune responses.
As a result, memory and cognitive dysfunction in Alzheimer’s dementia mice were recovered, and excessive accumulation of beta amyloid and tau protein in the brain was reduced.
Specifically, we identified changes in thyroid hormone levels in the blood and brain tissue of early (4 months old) dementia mice before extensive Aβ and Tau pathology occurred.
There was no significant difference in thyroid hormone levels in the blood between normal and dementia rats, but when comparing thyroid hormone levels in the brain, the activated thyroid hormone, triiodothyronine (T3), was found in dementia rats more than normal rats in the hippocampus area. ) was confirmed to decrease.
In addition, compared to normal rats, type 2 deiodinases (DIO2), which converts the inactive precursor hormone, thyroxine (T4) into the active hormone, T3, was decreased in the hippocampus of dementia rats. Confirmed.
We observed a decrease in DIO2 protein expression depending on the severity of the lesion in brain organoid models derived from Alzheimer’s disease patients and in postmortem brain tissue of Alzheimer’s disease patients.
Single-cell gene expression analysis was performed on the hippocampus of rats completely depleted of thyroid hormones by feeding an iodine-deficient diet for 10 weeks, and it was found that among the brain cell types, only microglia, which are responsible for immune function, showed significant changes due to the iodine-deficient diet. Confirmed.
In particular, microglial cells in mice deficient in thyroid hormones had a greatly reduced phagocytosis once morest beta-amyloid due to activation of a spontaneous inflammatory response. As a result, cognitive decline accelerated along with an increase in amyloid plaques in the hippocampus of thyroid hormone-deficient dementia mice.
Professor Inhee Muk said, “This study reveals that thyroid hormones play a role in forming the immune response of microglial cells once morest beta-amyloid in the brain,” and “It suggests the possibility of treating Alzheimer’s disease through thyroid hormone supplementation.” “We were able to do it,” he explained the significance of the study.
In a situation where there is a lot of controversy regarding the correlation between thyroid hormone imbalance and Alzheimer’s disease, this study confirms the decrease in thyroid hormones in Alzheimer’s disease in the brain and clarifies the metabolic disorders of thyroid hormones and their role in microglial cells, making them effective thyroid hormones. This is considered an important advancement in Alzheimer’s disease treatment research in that it suggests hormone therapy.
This research was conducted with support from the Korea Dementia Research Center (KDRC), funded by the Ministry of Science and ICT and the Ministry of Health and Welfare. was published in ‘Advances’
1710724046
#Thyroid #hormone #deficiency #brain #accelerates #pathological #phenomenon #Alzheimers #disease #Circulatorycerebrovascular #Medical #school #hospital #Hospitalpractice #center #Text #article