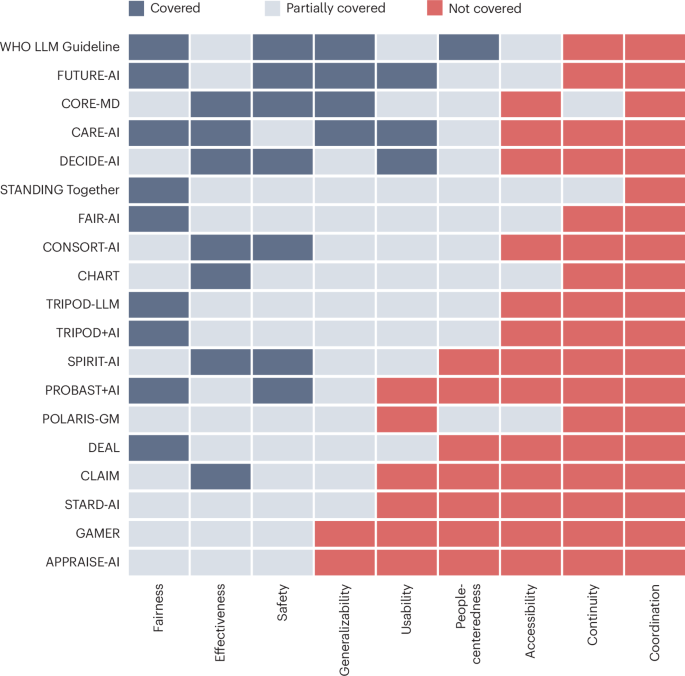
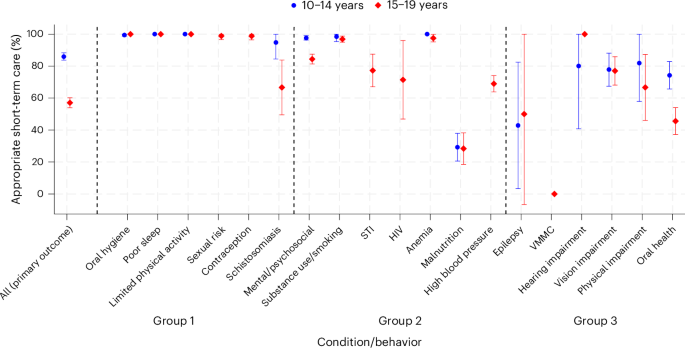
The pursuit of medical advancements relies heavily on clinical research, yet historically, these studies haven’t always reflected the diversity of the populations they aim to serve. A growing movement is now focused on embedding equity into the very governance of clinical trials, ensuring that research benefits everyone, not just select groups. This shift isn’t merely about inclusivity; it’s about improving the scientific rigor and real-world applicability of medical findings. Researchers and legal scholars are collaborating to create frameworks for auditable representation across trials and data infrastructures, addressing systemic biases that have long plagued the field of medical research.
A new governance blueprint, dubbed “Inclusion by Design,” aims to address these longstanding issues. Developed by a multidisciplinary team including researchers from the University of Modena and Reggio Emilia (UNIMORE) in Italy, and legal experts from South Africa and Switzerland, the framework focuses on ensuring representation across all stages of clinical trials and the data they generate. This approach recognizes that equitable research isn’t simply a matter of adding diverse participants; it requires a fundamental rethinking of how trials are designed, conducted, and analyzed. The goal is to move beyond simply acknowledging the necessitate for diversity to actively building it into the core of research governance.
The Need for Systemic Change
For decades, clinical trials have disproportionately included participants of European descent, leading to potential disparities in treatment effectiveness and safety for other populations. This bias stems from a complex interplay of factors, including historical inequities in healthcare access, socioeconomic barriers to participation, and a lack of trust in the medical system among underrepresented communities. As highlighted in recent publications, including work from the European Heart Journal [Cherubini, A. Et al. Eur. Heart J. 44, 3201–3210 (2023).](https://pubmed.ncbi.nlm.nih.gov/37215491/), addressing these biases is crucial for ensuring that medical advancements benefit all.
Johanna M. C. Blom, Associate Professor in Psychobiology and Pediatric and Behavioral Neuroscience at UNIMORE, and a key contributor to the “Inclusion by Design” framework, emphasizes the importance of proactive measures. “We need to move beyond simply acknowledging the problem and start implementing concrete strategies to ensure equitable representation in clinical research,” Blom stated in materials related to the framework. The framework proposes a system of auditable representation, allowing for transparent monitoring of diversity metrics throughout the research process.
Key Components of the “Inclusion by Design” Framework
The “Inclusion by Design” framework encompasses several key elements. First, it advocates for the integration of diversity, equity, and inclusion (DEI) considerations into the very design of clinical trials. This includes proactively identifying and addressing potential barriers to participation for underrepresented groups. Second, it emphasizes the importance of data infrastructure that supports the collection and analysis of demographic data, allowing researchers to identify and address disparities in outcomes. Third, the framework calls for increased transparency and accountability in research governance, with clear metrics for measuring progress towards equitable representation.
The framework also acknowledges the evolving legal landscape surrounding data privacy and artificial intelligence. The European Health Data Space, as proposed by the European Commission in 2022 [EC. Proposal for a Regulation on the European Health Data Space (COM(2022) 197 final) (2022).], and the EU Artificial Intelligence Act [EU. Artificial Intelligence Act (Regulation (EU) 2024/1689) (2024).], both have implications for how clinical data is collected, shared, and used. The “Inclusion by Design” framework seeks to navigate these complexities whereas upholding the principles of equity and inclusivity.
International Collaboration and Future Directions
The development of the “Inclusion by Design” framework is a testament to the power of international collaboration. Researchers and legal scholars from Italy, South Africa, Switzerland, and the United States have come together to address a global challenge. Ciara Staunton, from the School of Law at the University of KwaZulu-Natal in Durban, South Africa, brought a critical legal perspective to the project, ensuring that the framework aligns with ethical and regulatory standards. Sophie Tascedda, from the University Hospital Basel in Switzerland, contributed expertise in biomedical engineering and data science.
Looking ahead, the implementation of the “Inclusion by Design” framework will require a concerted effort from researchers, policymakers, and the pharmaceutical industry. The FACILITATE Consortium’s recent white paper on the return of individual participant data by design [FACILITATE Consortium. White Paper on the Return of Individual Participant Data by Design (2024).] highlights the importance of data sharing and transparency in promoting equitable research. Continued dialogue and collaboration will be essential to ensure that clinical research truly benefits all members of society. The ongoing work at UNIMORE, led by Blom and Pani, continues to refine and promote these crucial principles.
The push for equitable clinical research governance is not simply a matter of fairness; it’s a matter of scientific integrity and public health. As research continues to evolve, prioritizing inclusivity will be paramount to ensuring that medical advancements are effective and accessible to everyone.
What are your thoughts on the role of regulation in promoting equitable clinical research? Share your perspective in the comments below.
Disclaimer: This article provides informational content and should not be considered medical advice. Consult with a qualified healthcare professional for any health concerns or before making any decisions related to your health or treatment.