Photo caption: Rwanda’s Minister of State for Health, Yvan Butera, actively participated in the open-label clinical trial by receiving Sabin’s investigational vaccine, highlighting the collaboration in combating the Marburg virus outbreak.
WASHINGTON, Oct. 31, 2024 (GLOBE NEWSWIRE) — The Sabin Vaccine Institute has intensified its efforts with Rwanda to combat the ongoing Marburg virus outbreak by sending around 1,000 additional investigational vaccine doses. These doses are part of a randomized clinical trial aimed at expanding the scope of the ongoing open-label study, which has already seen more than 1,500 dedicated frontline workers vaccinated with Sabin’s vaccine.
Under a newly revised protocol sponsored by the Rwanda Biomedical Center, approximately 1,000 individuals identified as at-risk, including those working in mining, will participate in this crucial clinical trial. Participants will be randomly assigned in a 1-to-1 ratio, allowing one group to receive the investigational single-dose vaccine immediately, while the other group will get vaccinated 21 days later, a timing strategy aligned with the disease’s incubation period.
Genomic sequencing data suggests that the index case in Rwanda has a zoonotic origin, indicating the presence of viral strains resembling those typically found in fruit bats within a mine, shedding light on the outbreak’s potential sources.
The new trial arm will rigorously evaluate the vaccine’s safety, immunogenicity, and efficacy. Sabin is poised to deliver additional doses at the request of Rwandan officials, pending authorization from the U.S. Administration for Strategic Response and Preparedness, underlining the urgency and coordination in the response efforts.
“As Rwandan health officials determined, the most expeditious and effective way to reach this new group of people impacted by the outbreak is to adapt the current protocol,” says Sabin CEO Amy Finan. “While this vaccine is still investigational, our mission is to ensure that knowledge can move swiftly from research to real-world solutions, with scientific rigor and safety as our highest priorities.”
Over 1,700 vaccine doses have been delivered to Rwanda, with the first shipment arriving just nine days after the outbreak was officially declared on September 27. The initial trial phase focused predominantly on health workers, who have been among the hardest hit by the outbreak.
Sabin’s investigational vaccine is designed to prevent illness before exposure occurs, although it has not yet demonstrated clinical benefits for those who receive it. The candidate is currently undergoing Phase 2 trials in Uganda and Kenya, where no safety concerns have been identified thus far. In non-human primates, the vaccine has shown to confer rapid immunity within just one week of administration, while Phase 1 trials have confirmed its safety and immunogenicity in human subjects.
Marburg virus disease continues to pose a dire threat globally, characterized by a high case fatality rate and an absence of approved vaccines. Symptoms usually manifest within a window of two to 21 days following exposure to the virus.
Since 2019, Sabin has spearheaded vaccination efforts against filoviruses utilizing the cAd3 platform, and interim results from the Marburg trial are anticipated next year, with plans for a U.S.-based trial set for 2025. Moreover, Sabin plays a pivotal role in the MARVAC initiative, coordinated by WHO, which fosters global collaboration for the advancement of Marburg vaccine development.
About the Sabin Vaccine Institute
The Sabin Vaccine Institute stands as a leading advocate for the expansion of global vaccine access and usage, continually driving progress in vaccine research and development. Its mission includes amplifying vaccine knowledge and innovation. Through strategic partnerships, Sabin has cultivated a comprehensive ecosystem comprising funders, innovators, practitioners, and policymakers, all working towards the vision of a future free from preventable diseases. As a non-profit with over three decades of experience, Sabin is unwavering in its commitment to finding lasting solutions and ensuring that the benefits of vaccines are accessible to all, irrespective of their background or location. At Sabin, we wholeheartedly believe in the transformative power of vaccines. For further information, please visit www.sabin.org and engage with us on X, @SabinVaccine.
Media Contact:
Monika Guttman
Media Relations Specialist
Sabin Vaccine Institute
+1 (202) 662-1841
[email protected]
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f38d0939-533d-4fea-8d71-882be4461b28

The Marvel of Vaccines: A Cheeky Look at Sabin’s Marburg Trial in Rwanda
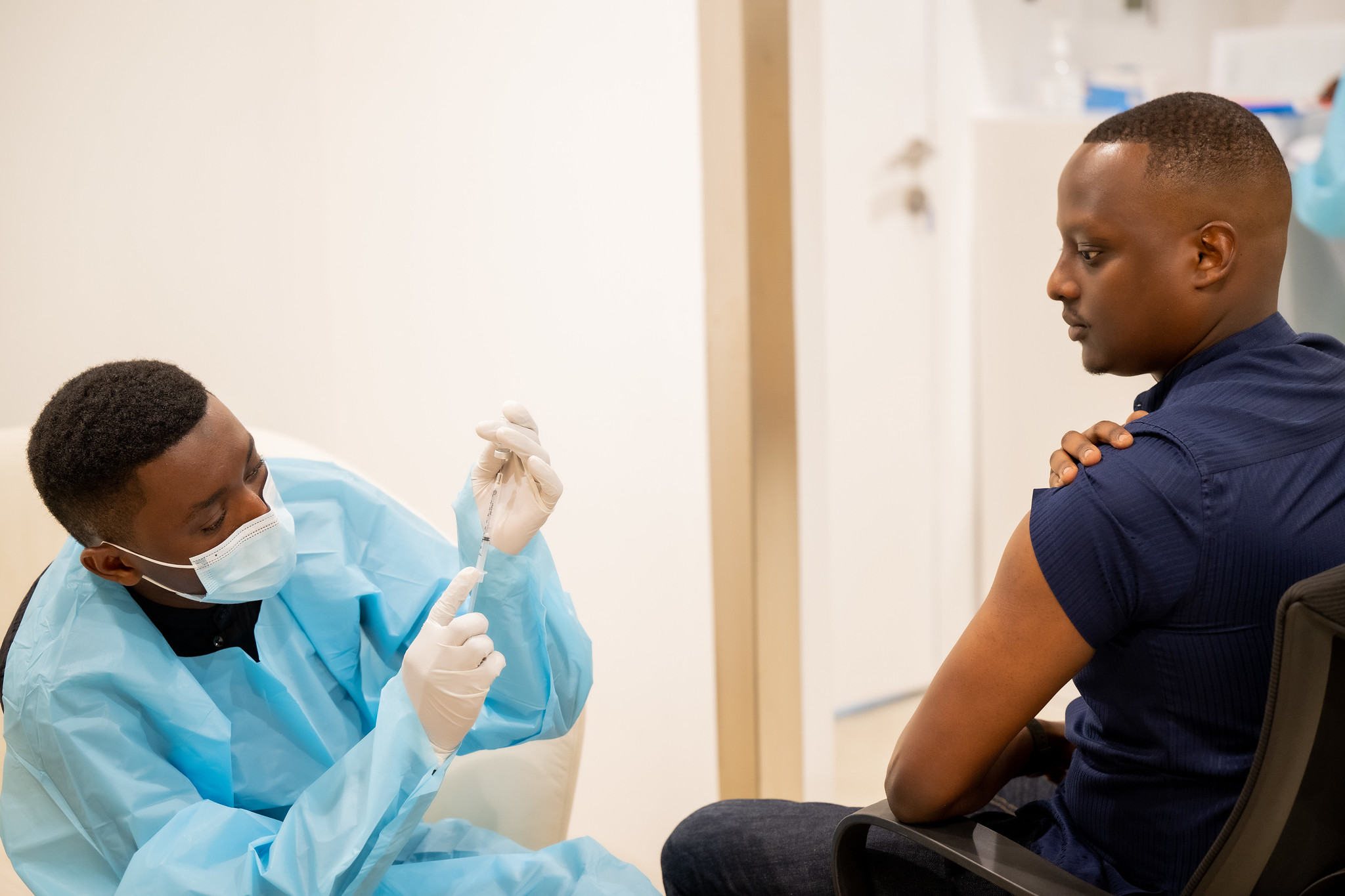
Photo caption: Rwanda’s Minister of State for Health, Yvan Butera, rolling up his sleeves for science!
Well, well, well, brave souls of Rwanda! While most of us are just trying to avoid sneezing on public transport, the valiant folks over there are stepping up to take investigational vaccines to combat the Marburg virus. Yeah, you heard that right – it’s not just your run-of-the-mill flu shot anymore – we’re talking about a vaccine that sounds like it should come with a superhero cape!
In a delightful twist of international collaboration, the Sabin Vaccine Institute has delivered around 1,000 doses of their investigational vaccine. This is part of a randomized clinical trial which certainly sounds more high-tech than whatever gadgets you still have lying around from the 90s! Frontline workers, who have already been vaccinated, are feeling even more like the superheroes they truly are.
Now, let’s break down this intriguing protocol – 1,000 at-risk individuals, including mine workers (cue the jokes about gold diggers!), will now be receiving this vaccine in a one-to-one randomization. Half will get it immediately and the other half will need to wait just a bit longer. 21 days later, they’ll finally get their own dose. I mean, let’s not rush into things; the only thing worse than waiting for a bus is waiting for a vaccine, am I right?
Why the Marburg Virus Matters
Genomic sequencing is all the rage these days – and for good reason! Turns out, the Marburg virus has a rather unglamorous history, with strains similar to those found in fruit bats. Seriously, when was the last time you said “I want to be like a fruit bat?” Much less a bat that brings death!
You see, Marburg is no laughing matter. With a high case fatality rate, it’s playing hardball with humanity, and the stakes are serious. As they say, ‘the only thing necessary for the triumph of evil is for good men to do nothing’— and here, the good folks of Rwanda are showing they mean business!
The Sabin Strategy
Leading the charge, Sabin CEO Amy Finan chimed in with her take on this matter: “While this vaccine is still investigational, our mission is to ensure that knowledge can move swiftly from research to real-world solutions…” Can we just take a moment? This is what superhero scientists sound like! You might expect her to wear a lab coat, but I reckon she’s also got a spandex suit tucked away somewhere.
It’s a relief to know that more vaccines are on their way following a request from Rwandan officials. After all, nothing says “I care” like a batch of vaccine doses and a safety protocol so thorough it could make even the most nervy person feel like they’re in the hands of pros.
Conclusion: A Future Free From Viruses?
The fight against the Marburg virus continues with Sabin’s efforts promising interim results expected next year. Until then, the world watches, holding its breath and maybe hoping that fame doesn’t come calling for fruit bats anytime soon. Paging Dr. Batman!
So there you have it, folks. As the Sabin Vaccine Institute pushes forward, we kind of have to wonder: if they can handle this, what more can they do? Perhaps next vaccine will be tailored for the next viral supervillain lurking in the shadows – stay tuned and keep those sleeves rolled up!
For more information, visit www.sabin.org and follow their updates because let’s face it, we’re in for an educational ride!
E this vaccine is still investigational, our mission is to ensure that knowledge can move swiftly from research to real-world solutions, with scientific rigor and safety as our highest priorities.” She’s basically saying they’re trying to turn nerdy medical research into a superhero story for the good of humanity!
With over 1,700 doses already delivered—just nine days after the outbreak was officially declared on September 27—it’s clear Sabin isn’t playing around. They’ve prioritized those on the front lines, like health workers, who are taking on Marburg like it’s their job…oh wait, it is.
Now, let’s not forget that the Sabin investigational vaccine is designed to prevent illness—before you even come within spitting distance of the nasty virus. It may not be perfect yet, but it shows promise in pre-clinical stages and early human trials. Basically, if the vaccine was a student, it’d be that one who always raises their hand and has its homework done on time. Who wouldn’t want that in their corner?
The Marburg virus disease not only continues to pose a global threat with its terrifyingly high case fatality rate, but it also shows no mercy with an incubation period that can stretch from two to 21 days. So, it’s clear that vaccines are not just beneficial; they are downright essential.
In closing, as Sabin spearheads vaccination efforts using the cAd3 platform and gears up for more extensive trials, we can only hope that our intrepid researchers, health workers, and brave participants will succeed in outsmarting a virus that’s been crashing the party for far too long. We’re rooting for them! So here’s to the ongoing fight against Marburg, armed with investigational vaccines and a little bit of Rwandan determination. And who knows? Maybe one day, those brave men and women will be the superheroes of a rich new narrative in vaccine history!

:max_bytes(150000):strip_icc()/connie-boss-alexander-stephen-twitch-boss-010925-ba7c78b2a1cc49bcb039b4a2473f08dd.jpg)

