Let’s talk regarding our enemy – tooth decay.
The chemical story of tooth decay
There are two main reasons for tooth decay. Tooth decay bacteria mentioned earlier is one of the factors, and the specific name of tooth decay bacteria is “Streptococcus transglycansIt is said that this bacterium is often transmitted by adults before children are regarding three years old. The reasons include using chopsticks and spoons used by parents, or taking turns drinking drinks; another factor is the sugar contained in food, the main component is “sucrose”. “.
When these two factors combine, the following happens: First, S. transglycans uses sucrose to make a molecule called “glucan.” The chemical formula of glucan is (C6H10O5)n, which will be explained in detail later. The glucan attaches to the tooth surface and becomes a habitat for Streptococcus transglycans. In addition, other bacteria in the mouth (according to statistics, there are more than 600 kinds of bacteria in the mouth) are also mixed into it.
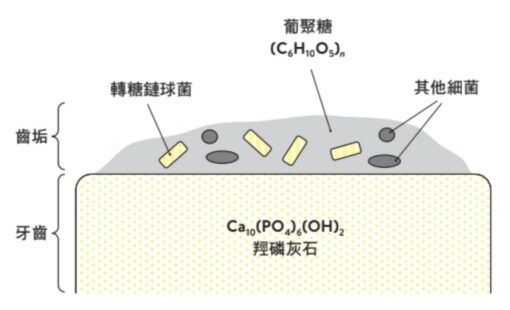

These compositions that adhere to the teeth are called “Plaque, also sometimes referred to as “tartar” or “biofilm” (also known as biofilm). You may have heard these terms in commercials for toothpaste, etc.
Afterwards, Streptococcus transsaccharides, which gain habitat, produce large amounts of “acids” that trigger demineralization and ultimately lead to tooth decay. This process is shown below.
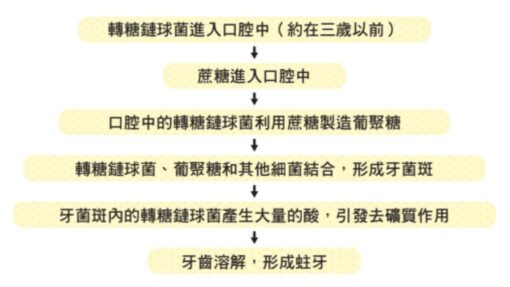

S. transglycans live in warm glucans and break down lactic acid; in fact, they also break down acetic acid, and an acid called formic acid (HCOOH), but lactic acid has a higher proportion. These acids trigger strong demineralization, dissolve teeth and cause cavities.
Before this happens, you must brush your teeth well to completely remove the plaque (glucan + bacteria) that sticks to the teeth! Even if you rinse your mouth, the sticky plaque is not easy to fall off. The most effective method is to brush your teeth well. Toothpaste contains abrasives (particles that help remove dirt), which can effectively remove sticky plaque.
Desserts that are not prone to tooth decay
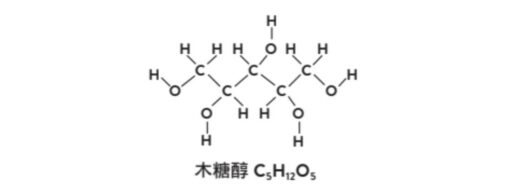
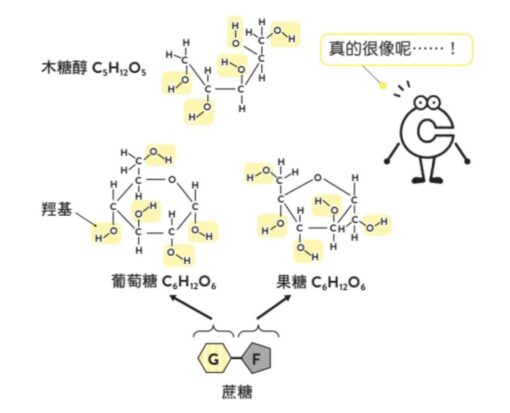
In the last unit we explained how sugar causes tooth decay. In fact, there are some molecules that taste as sweet as sugar, but are less likely to cause tooth decay. One of the most famous molecules is “xylitol”. You may have heard of chewing gum with xylitol! The chemical formula of this molecule is C5H12O5the detailed structure is shown in the figure below.

Why does xylitol taste sweet, but it is not easy to cause tooth decay? Before answering this question, let’s recall why sucrose (sugar) causes tooth decay. Sucrose is the material used by Streptococcus transsaccharides to make glucan, and the fructose produced during the reaction will be used as a source of nutrients by Streptococcus transsaccharides and decomposed into lactic acid molecules.
What regarding xylitol? First of all, unlike sucrose, xylitol is a material for making glucan, and Streptococcus transsaccharides does not use xylitol as a source of nutrients, so it will not decompose lactic acid. Therefore, although they taste sweet, they are not easy to cause tooth decay.
A problem arises here. The structures of xylitol and sucrose seem to be very different, but why are they also sweet? If you change the way of drawing xylitol a little like the picture below, you will find that its structure is very similar to glucose and fructose that make up sucrose, and it has many hydroxyl groups.



– This article is excerpted from“Everything in life can be written in chemical formulas”November 2021,happy culture。





