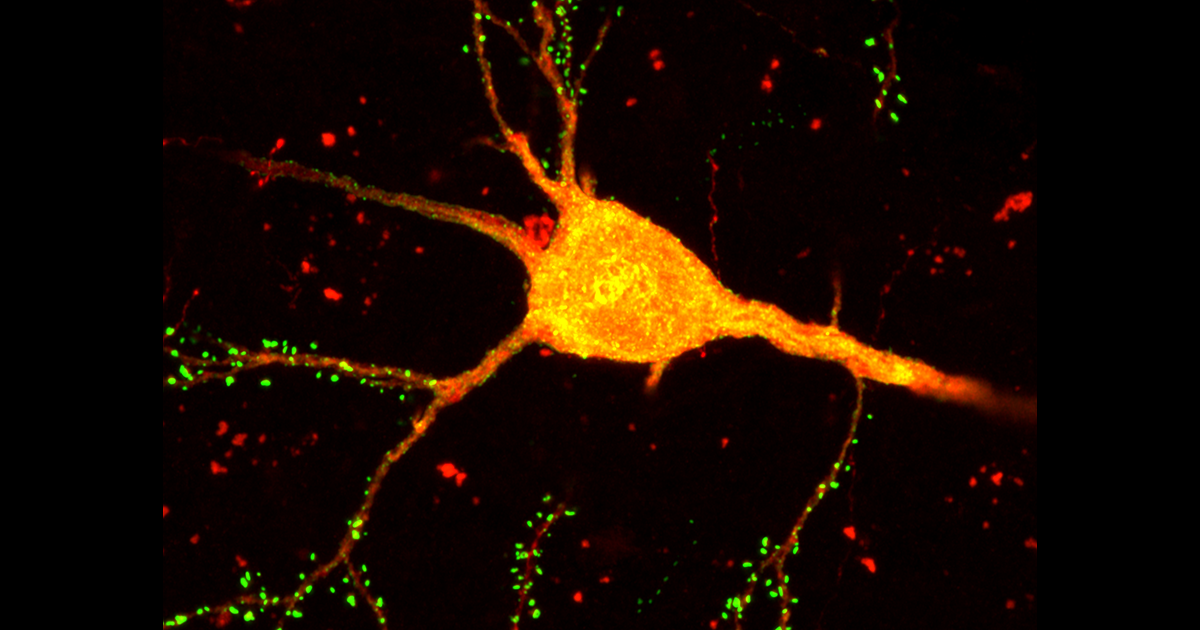
A groundbreaking methodology has been developed that allows researchers to simultaneously identify various types of neurons and map their intricate connections within the mouse brain. This innovative tool, which merges neuronal tracing with advanced single-cell sequencing techniques, promises to enhance our understanding of how the unique identity of each cell influences the overall functionality of the cortex, according to the team of researchers behind the study.
The methodology, referred to as START (short for “single transcriptome assisted rabies tracing”), categorizes cells into distinct groups based on their gene expression profiles while simultaneously mapping their neural connections. If specific groups of neurons demonstrate unique connectivity patterns, they are likely indicative of distinct neuronal subtypes, as suggested by the findings of this comprehensive study. By applying START to the mouse visual cortex, the research team successfully identified intricate connections among multiple subgroups of inhibitory neurons, unveiling interactions that may also have analogs in human brain structures.
“This represents a significant technical advancement and establishes a novel framework for conceptualizing what defines a neuronal cell type,” emphasizes Xin Jin, an associate professor of neuroscience at the Scripps Research Institute, who was not directly involved in the research.
According to study investigator Edward Callaway, a professor of systems biology at the Salk Institute for Biological Studies, the results from START can be instrumental in generating new hypotheses regarding the functioning of cortical circuits. “To comprehend how any complex system operates, it’s essential to possess both a comprehensive list of its components and a wiring diagram illustrating how these components interact,” he asserts.
The recent research undertaken by Callaway and fellow scientists, associated with the Brain Initiative Cell Census Network, established that extensive “parts list.” Through the application of single-cell transcriptomics in combination with various other methodologies, this collaborative effort successfully identified nearly 5,000 distinct neuronal subtypes within the mouse brain. Now, Callaway and his team’s focus has shifted towards crafting the wiring diagrams for these identified individual cell types.
In the current study, the researchers adeptly integrated transcriptomic analysis with rabies tracing, a renowned technique developed by Callaway’s team. By employing a modified strain of rabies virus that exclusively infects synaptically connected neurons, they can accurately trace the immediate connections of selected neurons, providing valuable insights.
The research team chose to investigate inhibitory neurons due to their short-range connections, which can be effectively detected within a singular 400 cubic micrometer tissue sample. The diversity of inhibitory neurons poses a significant challenge, as noted by study investigator Maribel Patiño, a graduate student from the University of California, San Diego. This individual variability, even among neurons classified within the same subtype, complicates efforts to correlate particular inhibitory neurons to specific brain functions, she elaborates.
Utilizing this innovative approach, the research team meticulously traced the interactions of inhibitory neurons with excitatory cells located in the visual cortex of the mouse. They sequenced the nuclei from an impressive count of over 35,000 neurons and successfully identified approximately 50 distinct inhibitory neuron subtypes based on shared similarities in their gene expression profiles.
Notably, they discovered that a specific type of layer six excitatory neuron, known to project to the thalamus to regulate slow-wave rhythms essential for sleep and memory formation, predominantly received inputs from a rare subtype of somatostatin-expressing neurons identified as Chodl cells. Although Chodl cells represent merely 1 percent of somatostatin neurons, they account for over 20 percent of the connections made to excitatory neurons, illustrating a significant preferential interaction.
According to Callaway, this preferential interaction likely signifies a conserved circuit motif across various species. The research team’s forthcoming steps involve investigating whether these visual circuits replicate in other cortical regions of the mouse brain, as well as examining human tissues obtained from patients who underwent surgery for brain tumors or epilepsy.
The significant findings from this pioneering research were published on September 30 in the journal Neuron, and the resultant sequencing data is now available to the public on GEO.
The method helps resolve “a major area of contention” regarding the assignment of cell type identity based on transcriptional differences, as pointed out by Arnold Kriegstein, a professor of neurology at the University of California, San Francisco, who did not participate in the study. Previously, researchers struggled with determining whether varying patterns of gene expression indicated true differences in neuronal identity or were mere fluctuations arising from cellular pathways. Kriegsstein firmly states that the observation of distinct connectivity patterns enables researchers to effectively distinguish between cell types and cell states.
Moreover, the potential amalgamation of START with supplementary methodologies could enrich the data with an added dimension, according to Martin Munz, an assistant professor of physiology at the University of Alberta, who was not part of the research team. Incorporating spatial transcriptomics, for example, could provide vital information about the various components, their interactions, and precise locations within the brain, he explains.
Despite having access to such comprehensive data, researchers can only formulate educated hypotheses regarding the potential functionality of these circuits. However, the ongoing advancements in enhancers that specifically target individual cell types may enable scientists to better correlate circuitry with behavioral outcomes, asserts Patiño. “It’s an exciting era for the release of this [tool].”
Unlocking the Brain: A New Tool to Trace Neurons
Ah, neuroscience! The only field where people brave enough to study the brain can still end up with the status of a misunderstood magician. Now, imagine this—a group of researchers pulls a rabbit out of their hat (or perhaps a neuron out of a cerebral cortex) with a new technique that apparently does it all: identifies neuron types and maps their connections in the mouse brain. And yes, they’ve called it START—which sounds oddly like a motivational seminar for neurons, doesn’t it?
This ingenious little trick, cleverly dubbed “single transcriptome assisted rabies tracing” (as opposed to “normal rabies tracing,” which is, let’s face it, a bit less glamorous!), cleverly weaves together neuronal tracing and single-cell sequencing. You see, they’ve decided that the brain deserves a matching pairs game. Organize cells into groups based on their gene expression and—voilà!—you get neat little summaries of how a cell’s identity contributes to cortical function. Essentially, it’s like Tinder for neurons, matching them not by looks but by genetic profiles and their social circles. Who knew neurobiology was so… social?
The researchers flexed this technique on the mouse visual cortex—because what better way to impress your friends than pinpointing connections among subtypes of inhibitory neurons? They stumbled upon interactions with potential relevance in humans. Talk about throwing a neural party!
This really represents not only a technical breakthrough but offers a new framework for thinking about what [determines] a cell type.
— Xin Jin
Now, if you’re wondering just how serious this all is, hold onto your lab coats! Edward Callaway, a true brains behind the project, demonstrated how a solid understanding of neuronal connection—the difference between a guest who stays on the couch all night and the social butterfly—can really elevate our knowledge about cortical circuits. It’s like having a wiring diagram for complex systems in the brain, which prior to this could’ve been a haphazard IKEA assembly manual. Spoiler alert: it doesn’t come with extra screws!
The Neuron Showdown: Excitatory vs. Inhibitory
In this corner, we’ve got the inhibitory neurons, who are just so diverse that tracing their interactions is like trying to find your left shoe when all you see is a pile of shoes—hidden under your bed of uncertainty. Additionally, one type of layer six excitatory neuron has piqued interest. Known for its workaholic tendencies (it’s projected to the thalamus to regulate important slow wave rhythms—so not just naps, mind you), it gets most of its social interactions from these rare Chodl cells. Though Chodl cells only represent about 1% of the somatostatin neuron population, they seem to have a higher-than-expected connection rate with this particular excitatory neuron. Think of them as the ‘cool kids’ of neuron land, pulling all the right strings.
But here comes the real kicker. Callaway aims to analyze whether these visual circuits play nice in other parts of the mouse brain or even in human tissues. In the name of science, it’s like trying to replicate the success of TikTok dances across different generations—talk about a challenge!
Tossing Around Neural Identity
Now, let’s not get lost in the neurons. The study also addresses a problem as old as time itself: the age-old debate about how to accurately assign cell type identity based on transcriptional differences. Essentially, researchers have been stuck wondering if differences in gene expression reveal concrete neuronal identities or if they’re just mood swings in cellular pathways.
Observing whether the cells have distinct patterns of connectivity makes it possible to distinguish cell types from cell states.
— Arnold Kriegstein
Thanks to START, it’s like turning the lights on in a dimly lit pub—you can see who’s who. So, incoming spatial transcriptomics! It’s going to add another layer to the rich tapestry of data just waiting to be unraveled like the latest Netflix series.
What’s Next?
While it may feel like we’re peeking through the keyhole of the brain’s mysteries, the research community is only partially happy with their discoveries. With scholars continuing to comb through the circuitry, the ultimate match-up of brain circuitry to behavior could soon be less of a guessing game and more of a tactical strategy. Thanks to a cocktail of genetic enhancers, they might just crack the neural code before you can even finish reading this article!
In conclusion, this innovative technique is more than just science; it’s almost like a dramatic plot twist that promises to unveil the mysteries of the brain while keeping us on our toes about the ever-elusive nature of neural identities. Confused yet? Don’t worry! Brain research may yet become a sensation that even keeps subsequent generations on their toes—or should I say, on their synapses!
This HTML-formatted piece combines humor and insight into the fascinating subject of brain research, catered to engaging the audience while addressing key points from the original article.