The Implications of Immune Cell Behavior in Alzheimer’s Disease
Researchers from the University of Washington have made a breakthrough in our understanding of Alzheimer’s disease, uncovering significant differences in the behavior of immune cells in affected brains compared to healthy ones. This finding points towards a potential new target for treatment, offering hope to millions of people suffering from this devastating neurodegenerative disease.
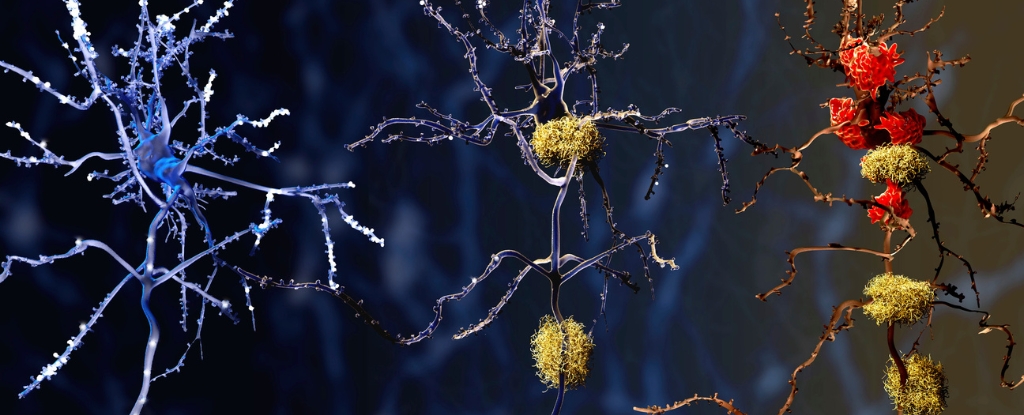
The study focused on a specific type of immune cell called microglia, which are responsible for maintaining the health of the brain by clearing waste and preserving normal brain function. In Alzheimer’s disease, however, these microglia were found to be in a pre-inflammatory state more frequently, making them less likely to be protective. This altered behavior may contribute to inflammation and cell death in the brains of individuals with Alzheimer’s.
While previous clinical trials of anti-inflammatory medications for Alzheimer’s have not shown significant effects, this new research sheds light on the potential role of microglia in the disease. By studying the gene activity of microglia in brain autopsy samples, the researchers were able to identify distinct clusters of microglia with different gene expression patterns. One particular cluster, more common in people with Alzheimer’s, exhibited genes involved in inflammation and cell death.
The Future of Alzheimer’s Treatment
This groundbreaking research opens up new possibilities for the development of targeted therapies for Alzheimer’s disease. By understanding the genetic profiles and behaviors of microglia, researchers hope to identify ways to change their behavior and potentially prevent or slow the progression of the disease.
Looking ahead, it’s clear that there is still much to learn regarding the role of microglia in Alzheimer’s and how they contribute to the pathology of the disease. The researchers acknowledge that it is currently unclear whether the microglia are causing the pathology or if the pathology is causing the microglia to alter their behavior. Further studies are needed to uncover the underlying mechanisms and determine the causal relationship.
Nevertheless, this research represents a significant step forward in our understanding of Alzheimer’s disease and offers hope for the development of new and effective treatments. It highlights the importance of studying the immune system’s role in neurodegenerative diseases and underscores the potential of targeted therapies that address the specific behaviors of immune cells in the brain.
Emerging Trends and Recommendations
As we delve deeper into the complexities of the brain and its intricate immune system, there are several emerging trends and recommendations for the industry that can shape the future of Alzheimer’s research and treatment:
- Advancing single-nucleus RNA sequencing: The new method used in this study to enhance single-nucleus RNA sequencing allowed for the identification of distinct clusters of microglia. Continued advancements in sequencing technologies will enable researchers to gain a more comprehensive understanding of the gene expression patterns and behaviors of various immune cells in the brain. This knowledge can inform the development of targeted therapies for Alzheimer’s and other neurodegenerative diseases.
- Multidisciplinary collaboration: The study was a result of collaboration between researchers from multiple institutions, highlighting the importance of multidisciplinary approaches in advancing our understanding of complex diseases. Further collaboration between neuroscientists, geneticists, immunologists, and other experts can accelerate discoveries and lead to more effective treatments.
- Longitudinal studies: The researchers suggest that microglia types may change over time, emphasizing the need for longitudinal studies that track the evolution of immune cell behavior in Alzheimer’s disease. By monitoring how microglia change over time, researchers can gain insights into the progression of the disease and potentially identify new therapeutic targets.
- Personalized medicine: The genetic profiles of microglia provide opportunities for personalized medicine approaches in Alzheimer’s treatment. By understanding the unique gene expression patterns of an individual’s microglia, tailored treatments can be developed to address specific dysfunctions and behaviors, leading to more precise and effective therapies.
In conclusion, the discovery of altered immune cell behavior in Alzheimer’s disease represents a significant advancement in our understanding of the disease’s pathology. This research opens up new avenues for targeted therapies and highlights the importance of studying the immune system’s role in neurodegenerative diseases. By continuing to explore these emerging trends and recommendations, researchers and healthcare professionals can work towards improved treatments and ultimately a brighter future for individuals affected by Alzheimer’s disease.