August 17, 2022
image source,Getty Images
The new coronavirus continues to mutate, and the Omicron variant BA.4 / BA.5 has become the dominant strain in many regions, and the persistence and upgrade of vaccine efficacy has attracted more attention.
The British government on August 15 approved a new-generation vaccine developed by Moderna as a booster for mass vaccination.
The vaccine, the first to be approved as a booster in the UK, is called Spikevax and is called a bivalent vaccine. Strain “BA.4/BA.5 can also provide stronger immune protection.
Hit two birds with one stone
Modena’s official website information showsthe results of the trial on 437 people showed that the upgraded version of the vaccine was safe, provided better immune protection once morest both the original virus and the newer Omicron subtype variant, and had the same side effects as the original vaccine.
The data showed that people who used the bivalent vaccine as a booster had high levels of antibodies in their blood and obtained stronger immune protection.
Moderna Spikevax uses the original new coronavirus strain and the Omicron variant BA.1 that appeared in early 2022, but the test results show that it can also provide Omicron subtype strains BA.4 and BA.5. Higher protection than older vaccines.
There is no evidence that bivalent vaccines perform differently in stopping the spread of the virus.
The British “Daily Telegraph” quoted the general manager of Moderna’s UK branch, Darius Hughes, as saying that the design of the Moderna mRNA vaccine provides room for adding other strains or variants in the future, which can be Flexible processing, adding new strains or variants on the basis of retaining the original virus strain.
He explained that the updated vaccine contains the genetic code for the original Omicron strain, BA.1, which also applies to BA.4 and BA.5.
Moderna hopes to develop a bivalent vaccine combined with the flu vaccine by next winter: the new crown-flu vaccine, and by the winter of 2024-2025 to develop a “three-in-one vaccine once morest the new crown, influenza and respiratory syncytial virus (RSV)” All-in-one vaccine.
Other pharmaceutical companies are also developing vaccines once morest Omicron and its subtypes, including Pfizer, Sinovac and Sinopharm.
image source,Getty Images
Experts in virology and epidemiology believe the virus has become more ‘cunning’ as the main reason people get infected multiple times
“Otto”: BA.4 and BA.5 are more “cunning”
The increased immune evasion of SARS-CoV-2 variants and their subtypes, coupled with reduced immunity in people, and a higher chance of infection among people who have never been infected with Covid-19 (20% of the UK population) have contributed to the risk of superinfection. A new wave of epidemics that is the main feature. Of particular concern are the newly emerged Omicron subtype strains BA.4 and BA.5.
BA.4 and BA.5 can “essentially escape” immune protection from vaccination or previous infection, meaning they have a strong ability to evade immune suppression, the New England Journal of Medicine reported.
A study in the journal Science also showed that the initial Omicron variant acts like a “stealth virus” that makes the immune system less vigilant and discriminating once morest later subtype variants. This is what scientists call “immunoblotting”.
While the recent wave of severe illnesses requiring hospitalization has not increased proportionately, infections can still cause disruption and inconvenience to daily life, pose additional risks to those with underlying medical conditions or are immune-compromised, and may increase the risk of developing “long-term” Corona” risk.
There’s no indication that the newest subtype variant is more dangerous than the original Omicron, but it’s impossible to say for sure because only laboratory and animal studies are available.
A Japanese study showed that BA.4 and BA.5 were more likely to grow in lung cells. The symptoms of hamster infection are more severe than those of early SARS-CoV-2 infection.
In terms of severity, the new coronavirus and its descendant variants may look more like the flu, but the difference, at least for now, is that the flu has only one peak season per year.
Vaccines: Upgrading and “Broad Spectrum Immunity”
As far as the new crown epidemic is concerned, vaccine upgrades and vaccination plans are still the key.
In addition to Moderna, several pharmaceutical companies are also upgrading the new crown vaccine and developing an updated version of the vaccine that is effective once morest Omicron, including Pfizer, Sinopharm, and Sinopharm.
Moderna’s “bivalent vaccine” Spikevax was the first to be approved by the British government as a booster.
However, it is difficult to judge how effective the updated vaccine is for the currently circulating subtype strains and the latest subtype strains or completely new mutant strains that may appear in the future.
In June 2022, two research reports published in “Nature” and “Science” respectively provided some clues for solving this problem from different aspects.
A report published by a Chinese research team in the journal “Nature” and a report by a British research team in the journal “Science” examined the causes of repeated infection with the new crown from different aspects.
image source,Getty Images
Current vaccines mainly target the spike protein that covers the surface of the new coronavirus.
A Chinese research team published on June 17A research paper in the journal NatureIt is said that the vaccine currently developed for the early variant of Omicron has been escaped by the emerging subtype, so it may not be able to produce effective immune protection once morest the emerging Omicron subtype variant.
This research was led by the team of Professor Xie Xiaoliang from Changping Laboratory of Beijing Future Genetic Diagnosis Advanced Innovation Center of Peking University, and participated by Tsinghua University, Nankai University, Academy of Social Sciences, Capital Medical College and other institutions.
The study found that following the vaccinated people were infected by the early Omicron strain BA.1, they produced antibodies that might neutralize the BA.1 virus and the original new coronavirus, and the subtypes that appeared later, including BA. 2.12.1, BA.4 and BA.5, all have mutations that escape these antibodies.
These subtype variants were found to have greater escape capacity for neutralizing antibodies in plasma from patients who received three doses of the vaccine and were infected with Omicron BA.1 post-vaccination.
Neutralizing antibodies inactivate the virus and help provide “broad-spectrum immune” protection.
image source,Getty Images
The team explained that the Omicron subtype variant can break through the humoral immunity (antibody immunity) protection caused by the Omicron virus itself, and the memory immunity generated by this breakthrough infection is mainly directed once morest the original strain.
Vaccines made with early mutants of Omicron have limited neutralization of emerging subtypes.
In this regard, the research team of Peking University believes that such vaccines may not be able to provide a “broad-spectrum” immune protection effect once morest the new Omicron subtype variant.
“Unlike when Omicron first appeared, the current Omicron subtype variants can target the humoral immunity (breakthrough infection) induced by Omicron itself, such as in Omicron following vaccination. Mikron is infected.”
Omicron’s breakthrough infection-induced memory immunity was primarily once morest the original SARS-CoV-2 strain, and “this in turn narrows the diversity of antibodies elicited and may further facilitate the emergence of future variants.”
image source,Archyde.com
Pfizer vaccine
Virus Discrimination and “Immunoprinting”
Imperial College London research team 14 Junepublished in the journal Science,It is said that Omicron infection does not necessarily enhance natural immunity to emerging virus variants and subtypes. The immune-enhancing effect of infection is more dependent on the individual’s infection history.
Studies have shown that an individual’s vaccination and infection history can have a huge impact on their immune response to variants, including Omicron.
This is because of the role of “immuno-imprinting”; the memory and immunity of the “imprinting” left by the initial infection to the virus that invaded at that time has nothing to do with Omicron and its subtype strains.
This goes once morest a common assumption. Infection with the virus is often thought to provide a natural boost of immunity, enabling a person to better recognize the variants encountered and defend once morest future infections, but new analysis finds that even people who have received three doses of the vaccine are still reinfected, Including re-infection of Omicron variant and its various subtypes.
The research team found that among those who had received three doses of the vaccine and had not previously been infected with the new crown, following infection with Omicron, the body’s immunity to the previous multiple mutant strains (Alpha, Beta, Gamma, Delta and the original virus strain) increased. Enhanced, but less immune-enhancing effect on Omicron itself.
People who recovered from the initial infection in the outbreak were later infected with the Omicron variant, and the first infection did not act as an immune booster.
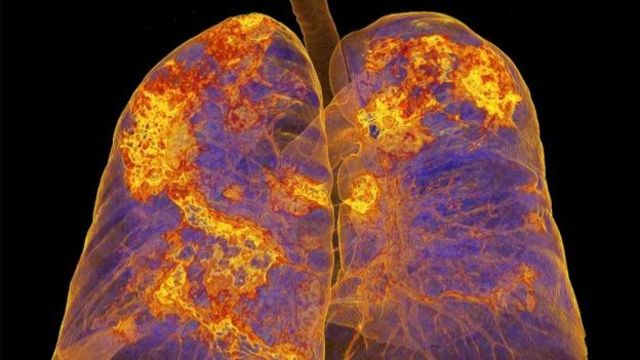
image source,SPL
Lungs of coronavirus patients
This is because the study found that T cells cannot make up for the poor recognition ability of Omicron antibodies. Even in patients infected with this mutant strain, T cells still have poor recognition of the spike antigen of the mutant strain and cannot provide effective protection.
Therefore, lead author of the report, Rosemary Boyton, a professor in the Department of Infectious Diseases at Imperial, explained that having been infected with Omicron once did not equate to a stronger immunity once morest re-infection.
“It is worrying that Omicron may mutate further into a more pathogenic strain, or become better able to overcome vaccine protection,” she said. In this case, people infected with Omicron It will be difficult to fight off future infections, depending on their immunosignature.”
One of the report’s authors, Dr Joseph Gibbons from Queen Mary University of London, explained: “The link between a person’s history of infection and their response to vaccines is now clear. Previous infection with different variant strains can affect the immune response. Potency and durability.”
However, they stress that it is certain that vaccination can provide sustained protection once morest severe illness and death, but the impact of infection and reinfection on long-term health, including “coronavirus,” is unclear.
image source,Getty Images
The efficacy of the new crown vaccine will gradually weaken following regarding half a year of vaccination, but the degree of protection is still high
How does immunity arise?
The immune system is the body’s defense system once morest infection and consists of two parts: the innate immune response and the adaptive immune response. The innate immune response does not learn and does not target any specific virus, and therefore does not develop immunity to the new coronavirus.
The adaptive immune response is more targeted, producing cells that target antibodies that can stick to the virus and block it, and T cells that recognize the virus and target infected cells, a cellular response.
research showsit takes regarding ten days to develop antibodies once morest the new coronavirus, and the sickest patients develop the strongest immune responses.
Preliminary studies have shown that there is no weakening of T cells and immune memory following vaccination with the new crown vaccine, but the latest research shows that the advantages and disadvantages of this feature for “broad-spectrum” immune protection need to be further explored.
image source,Archyde.com
Why are neutralizing antibodies more important?
The ribonucleic acid (RNA) of the COVID-19 coronavirus has spike proteins on its surface that can attach to certain human cells. Once the viral RNA enters the cell, it begins to replicate and initiate the production of proteins, causing the virus to infect more cells and spread throughout the body, especially the lungs.
The immune system responds to different parts of the virus, but the focus is on the spike protein, which recognizes it as foreign and automatically starts producing antibodies in response.
according toHarvard Medical School websiteExplain that neutralizing antibodies are antibodies produced by the body’s immune system that attack the coronavirus’ spike protein, making it harder for the virus to attach and enter human cells. Neutralizing antibodies can provide longer-lasting protection once morest reinfection than binding antibodies.
Man-made versions of neutralizing antibodies, called monoclonal antibodies, are approved for clinical treatment of COVID-19 in some countries, including the United States.



