Hidden dynamics: The proteins on the surface of the influenza virus are surprisingly mobile, new analyzes reveal. Accordingly, the viral neuraminidase can bend its “head” by almost 90 degrees and rotate around its axis. The second protein, haemagglutinin, on the other hand, shows a surprising “breathing”: Its three-pointed end opens and closes alternately. Both forms of movement expose previously unrecognized docking sites for antibodies – and thus have potential for medicine.
That Grippevirus is one of the most successful pathogens of all: this enveloped RNA virus repeatedly manages to form variants and adapt to new hosts. This regularly creates new influenza variants that can infect birds or spread to humans. The two play for their ability to evade the immune system and infect our cells out of the shell prominent surface proteins hemagglutinin (HA) and neuraminidase (NA) play a crucial role.
How these envelope proteins of the flu virus are structured and arranged has been largely clarified by virologists using cryo-electron microscopy, among other things. Until now, however, little was known regarding how these viral proteins move and what role this plays in infection, cell entry and the success of our immune system.
A virus of 160 million atoms
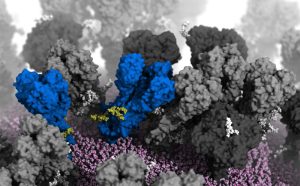
This is exactly what Lorenzo Casalino from the University of California in San Diego and his team have now clarified. On the basis of electron microscopic data and molecular analyzes of individual protein details, they created an atomic model of the entire H1N1 influenza virus including all envelope proteins. The 3D model included more than 160 million atoms, their positions and chemical bonds.
In a biophysical simulation, the researchers then determined how the envelope proteins behave and move under in vivo conditions – for example, when they have penetrated our airways and tissues. “Our simulations thus provide unprecedented dynamic views of the plastic conformation changes of the hemagglutinin and neuraminidase appendages,” say the team.
Neuraminidase nods its head
In fact, the simulations revealed three surprisingly dynamic movements of the viral envelope proteins. On the one hand, the neuraminidase protein can bend its distinctive “head” by more than 90 degrees. As a result, the protein head performs circular movements on its stalk. “Bending the head off exposes a previously unknown epitope on the underside of the neuraminidase head,” report Casalino and his colleagues.
The exciting thing regarding it: epitopes are potential docking sites for antibodies on the viral proteins. They therefore play a crucial role in how effectively the immune system can recognize and fight a virus, but also how well vaccines and antibody preparations work. “We have already identified a monoclonal antibody that can bind to the underside of the neuraminidase head,” the researchers report. This might open up new therapeutic options.
Inclination of the hemagglutinin exposes “old” docking sites
The same applies to the second protein movement identified in the simulation. Because the second envelope protein of the flu virus, haemagglutinin, can also tilt its tail slightly. This position is retained for a longer period of time – and immediately exposes several potential points of attack: “The gradual inclination of the HA ectodomain has a profound influence on the accessibility of the so-called ‘anchor epitopes’ at the lower end of the protein stalk,” say Casalino and his team .
These deep-seated docking sites are considered to be less susceptible to mutations and remain the same in many influenza subtypes. As a result, they can be attacked by broadly effective antibodies in the body or a preparation. “Constraining the hemagglutinin ectodomain in a tilted state might enhance the immune response to these anchor epitopes,” say the researchers. In fact, there are already several broadly neutralizing antibodies that fit these docking sites.
The protein head “breathes”
The third movement is much more unusual: the hemagglutinin protein “breathes”. The three-lobed end of this envelope protein periodically opens and closes. “The protein heads seem to breathe asymmetrically, they open and close independently of each other,” report Casalino and his colleagues. On average, at any given time, around 70 percent of the HA proteins in an influenza virus are closed and 30 percent are open.
Similar to the bending movements, this breathing of the envelope protein also makes the flu virus more vulnerable: When the head of the hemagglutinin opens, it exposes another potential docking site for antibodies in its center. According to the simulation, this epitope matches another potentially broad-spectrum antibody.
potential for medicine
According to the scientists, these new insights into the movements of the influenza envelope proteins are of considerable importance for medicine. “The plasticity of the protein conformations revealed in our simulations shows us weaknesses in the virus and exposes epitopes that would otherwise be hidden or inaccessible,” explain Casalino and his colleagues. This opens up new possibilities to attack these sites with tailor-made antibodies or vaccines.
It is particularly relevant that some of these potential docking sites remain the same even when new influenza variants are formed. That might make it possible to develop vaccines and flu drugs that don’t have to be adjusted every new season. (ACS Central Science, 2023; doi: 10.1021/acscentsci.2c00981)
Quelle: American Chemical Society
31 January 2023
– Nadja Podbregar


