The intricate relationship between genetics and brain structure is unlocking new frontiers in neuroscience. Recent large-scale studies are shedding light on how our DNA shapes critical brain regions responsible for memory, movement, and emotional regulation.These discoveries are not just academic—they hold the potential to transform our understanding of brain health and disease.
One of the most ambitious efforts in this field is led by the ENIGMA Consortium, a global collaboration of researchers dedicated to unraveling the genetic mysteries of the brain. Their latest findings, published in Nature Genetics,represent a important leap forward. By analyzing genetic and MRI data from nearly 75,000 individuals, the team identified 254 genetic markers linked to brain volume variations.
These genetic loci are associated with key subcortical regions, including the brainstem, hippocampus, and amygdala—areas crucial for functions like memory retention, emotional processing, and motor control. Remarkably, these genetic factors explain up to 35% of the variability in brain volume, offering unprecedented insights into the biological mechanisms that govern brain development.
Dr. Paul M. Thompson, a prominent figure in the ENIGMA project, emphasized the global significance of this research.”By conducting this research all over the world, we’re able to uncover patterns and connections that would be impossible to detect in smaller, isolated studies,” he said.This collaborative approach, involving 189 experts from 45 countries, ensures that the findings are robust and universally applicable.
This research goes beyond mapping genetic links—it delves into the biological processes that drive brain development and potential disorders. By understanding how genetic variations influence brain structure, scientists are paving the way for targeted therapies and early interventions for conditions like Alzheimer’s, Parkinson’s, and mental health disorders.
Unlocking the Genetic Secrets of Subcortical Brain Structures
Table of Contents
- 1. Unlocking the Genetic Secrets of Subcortical Brain Structures
- 2. Key Genetic Associations in Subcortical Brain Regions
- 3. Integrating Single-cell RNA Sequencing with GWAS
- 4. Implications for Neuropsychiatric and Neurological Disorders
- 5. What This Means for Future Research
- 6. Potential Implications for personalized Medicine Approaches
- 7. Unlocking the Genetic Secrets of Brain Development and disorders
- 8. The Genetic Architecture of Subcortical brain Regions
- 9. Bridging Genetics and Cellular Biology
- 10. Implications for Neuropsychiatric and Neurological Disorders
- 11. Future Directions in Brain Research
- 12. The Role of Lifestyle in Brain Health
- 13. Revolutionizing Brain Health: How Genetics is Shaping the Future of Medicine
- 14. The Power of Diversity in Genetic Research
- 15. Unlocking the secrets of Brain Structure
- 16. From Diagnosis to Treatment: A New Era of Medicine
- 17. Looking Ahead: The future of Brain Health
- 18. Unlocking the Genetic Secrets of the Brain: A New Era for Personalized Medicine
- 19. genetic Associations in Subcortical Brain Regions
- 20. Advanced Tools and Techniques Driving Discovery
- 21. Integrating Single-Cell RNA Sequencing with GWAS
- 22. Implications for Neuropsychiatric and Neurological Disorders
- 23. Future Research Directions
- 24. The Interplay Between Genetics and Lifestyle
- 25. Conclusion
- 26. Unlocking the Secrets of Brain Volume: The Role of Genetic Research
- 27. Why This Research Matters
- 28. what’s Next in Brain Research?
- 29. Key Takeaways
- 30. How do genetic variations identified in genes like CRHR1, MAPT, and ZNF786 contribute to variations in subcortical brain volumes?
- 31. The Genetic Blueprint of Brain Volume
- 32. Cutting-Edge Tools Driving Discovery
- 33. Implications for Brain Disorders
- 34. The Role of Lifestyle in Brain Health
- 35. Future Directions in brain Research
- 36. Conclusion
Table of contents
- 1. Unlocking the Genetic Secrets of Subcortical Brain Structures
- 2. Key Genetic Associations in Subcortical Brain Regions
- 3. Integrating Single-cell RNA Sequencing with GWAS
- 4. Implications for Neuropsychiatric and Neurological Disorders
- 5. What this Means for Future Research
- 6. Potential Implications for Personalized Medicine Approaches
Recent advancements in neuroscience have unveiled the complex genetic blueprint of subcortical brain regions, which are essential for functions like learning, memory, motor control, and emotional regulation. A landmark study has identified specific genes and cell types that considerably influence the development of these brain structures, offering fresh perspectives on their role in neurological health and disease.

Key Genetic Associations in Subcortical Brain Regions
The research highlighted significant genetic links to subcortical brain structures, revealing how variations in certain genes can influence their size and function. These findings provide a deeper understanding of the biological mechanisms underlying brain development and how they may contribute to conditions like Alzheimer’s, Parkinson’s, and schizophrenia.
Integrating Single-cell RNA Sequencing with GWAS
By combining single-cell RNA sequencing with genome-wide association studies (GWAS), researchers were able to pinpoint the specific cell types and genes involved in shaping subcortical regions. This innovative approach has opened new avenues for exploring the molecular foundations of brain structure and function.
Implications for Neuropsychiatric and Neurological Disorders
The study’s findings have profound implications for understanding and treating neuropsychiatric and neurological disorders.By identifying the genetic and cellular factors that influence subcortical brain regions, researchers are now better equipped to develop targeted therapies for conditions like depression, anxiety, and epilepsy.
What This Means for Future Research
This research marks a significant step forward in neuroscience, paving the way for more precise investigations into the genetic and cellular underpinnings of brain health. Future studies could leverage these insights to develop personalized treatments and preventive strategies for a wide range of neurological and psychiatric conditions.
Potential Implications for personalized Medicine Approaches
The discovery of genetic associations in subcortical brain regions holds immense promise for personalized medicine. By tailoring treatments based on an individual’s genetic profile, healthcare providers could improve outcomes for patients with neurological and psychiatric disorders.This approach could revolutionize how we diagnose, treat, and prevent these conditions, offering hope for more effective and individualized care.
Unlocking the Genetic Secrets of Brain Development and disorders
Recent advancements in neuroscience have shed light on the intricate relationship between genetics and brain structure. By exploring subcortical brain regions, researchers have identified key genes and cell types that play a pivotal role in brain volume variation. These discoveries not only enhance our understanding of brain development but also offer promising avenues for addressing neurological and psychiatric disorders.
The Genetic Architecture of Subcortical brain Regions
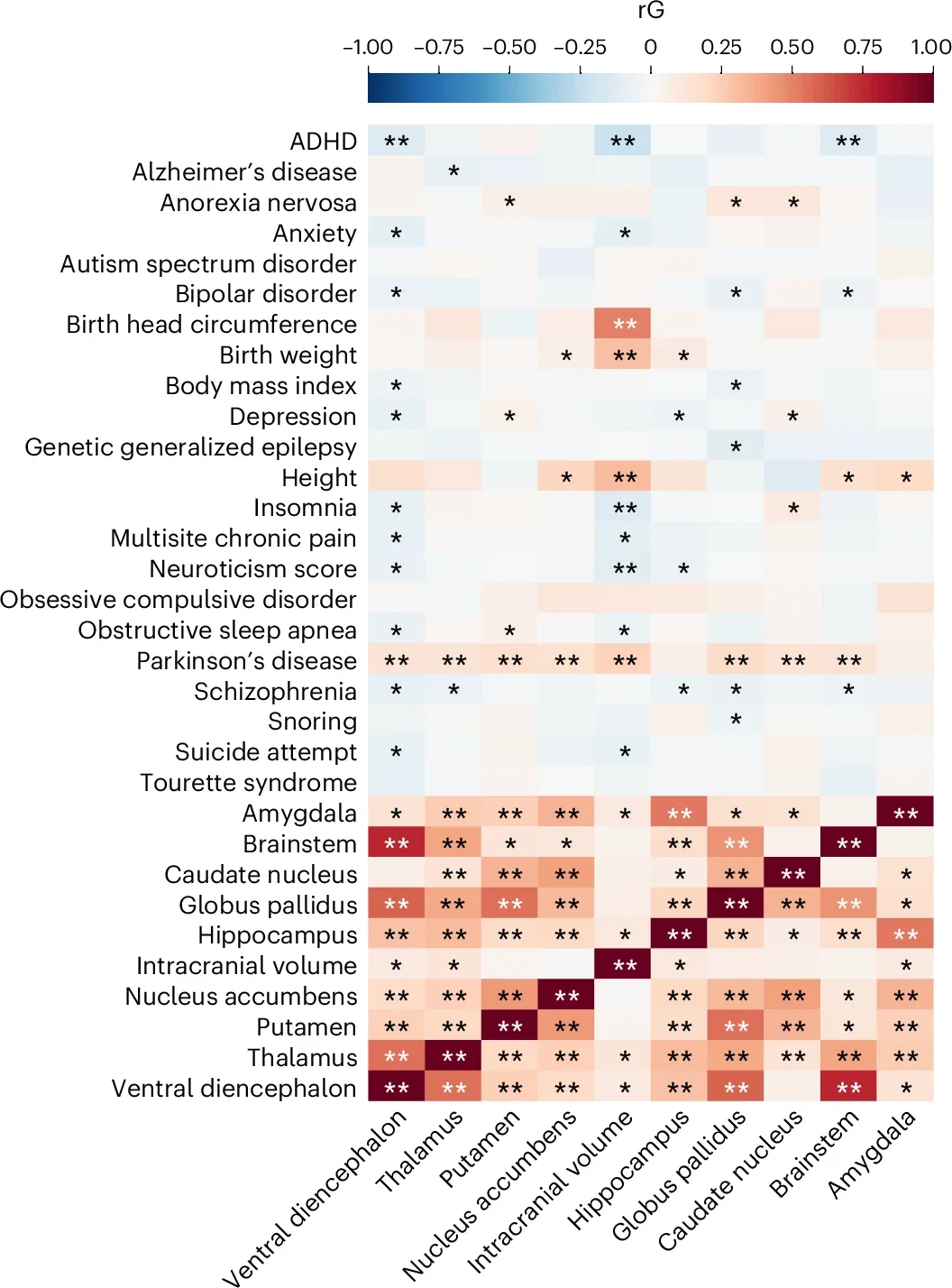
Studies have revealed distinct genetic associations across various subcortical regions.The brainstem,for instance,exhibits the highest number of autonomous genetic links,while the amygdala shows the fewest. Key genes such as CRHR1, MAPT, and ZNF786 have been linked to brain volume, influencing processes like intracellular signaling, tau pathology, and vascular resistance.
Advanced tools like MAGMA and transcriptome-wide association studies (TWAS) have further refined these insights. For example, the FOXO3 gene has been found to impact multiple brain structures, highlighting its broad role in brain morphology. similarly, genes from the WNT family are associated with the brainstem and ventral diencephalon, suggesting potential therapeutic targets for brain disorders linked to structural changes.
Bridging Genetics and Cellular Biology
By integrating single-cell RNA sequencing with genome-wide association studies (GWAS), researchers have identified specific cell types that contribute to brain volume variation. Dopaminergic neurons and astrocyte-like cells, as a notable example, are crucial to developmental processes. This integration provides a clearer picture of how genetic differences translate into structural changes during early brain development.
Implications for Neuropsychiatric and Neurological Disorders
The genetic foundations of subcortical brain volumes extend beyond structural analysis. These regions are frequently implicated in conditions such as Parkinson’s disease, ADHD, and other developmental or psychiatric disorders.For example, the basal ganglia—a region essential for motor control—has shown associations with genetic variants linked to Parkinson’s disease.
“Subcortical structures are frequently implicated in conditions like Parkinson’s disease, attention-deficit/hyperactivity disorder (ADHD), and other developmental and psychiatric disorders.”
Future Directions in Brain Research
These findings open new avenues for understanding the genetic basis of brain structure and function. By pinpointing specific genes and cell types involved in subcortical brain development, researchers can explore targeted interventions for brain disorders. Additionally, the combination of single-cell RNA sequencing with GWAS offers a powerful framework for future studies, enabling a deeper understanding of how genetic variations influence brain health.
As neuroscience continues to unravel the complexities of the human brain, discoveries like these bring us closer to unlocking the secrets of our most vital organ. Each breakthrough not only enhances our understanding of brain function but also offers new hope for treating and preventing neurological and psychiatric conditions.
The Role of Lifestyle in Brain Health
Beyond genetics, emerging research highlights the interplay between our genes and lifestyle choices. Factors such as diet, physical activity, and exposure to environmental toxins can significantly influence how genetic predispositions manifest. This dynamic interaction underscores the importance of daily habits in shaping neurological health,offering a holistic approach to brain wellness.
Revolutionizing Brain Health: How Genetics is Shaping the Future of Medicine
In a groundbreaking leap forward, scientists are uncovering the intricate connections between genetics and brain health. By analyzing genetic data from diverse global populations, including the UK Biobank, CHARGE, and the Adolescent Brain Cognitive Development (ABCD) study, researchers are gaining unprecedented insights into how our genes influence brain structure and function.These discoveries are not only reshaping our understanding of the brain but also paving the way for personalized treatments for neurological and psychiatric conditions.
The Power of Diversity in Genetic Research
One of the most remarkable aspects of this research is its global scope. By incorporating data from a wide range of populations, scientists are ensuring that their findings are inclusive and applicable to people worldwide. This diversity strengthens the reliability of the results and opens the door to medical solutions tailored to individual genetic profiles. As one expert aptly put it, “this paper, for the first time, pinpoints exactly where these genes act in the brain. This provides the beginnings of a roadmap for where to intervene.”
This inclusive approach is critical for advancing our understanding of how genetics interacts with environmental factors.By leveraging data from global initiatives, researchers can offer a more extensive view of brain health, ultimately leading to more effective and personalized interventions.
Unlocking the secrets of Brain Structure
The study focuses on identifying genetic variants linked to brain volume,a key factor in understanding brain health.By pinpointing specific genes and their roles in brain structure, scientists are uncovering potential pathways for diagnosing and treating conditions like Alzheimer’s, Parkinson’s, and other neurodegenerative diseases. For instance, understanding how certain genes influence brain aging could lead to interventions that slow or even prevent cognitive decline.
While the findings are groundbreaking, they remain correlational, leaving room for further investigation to establish definitive causal relationships. One especially promising avenue is the integration of single-cell RNA sequencing with genome-wide association studies (GWAS).This method has identified specific cell types, such as dopaminergic neurons and astrocyte-like cells, as significant contributors to variations in brain volume. Unraveling the precise roles these cells play within genetic pathways could pave the way for targeted therapeutic strategies.
From Diagnosis to Treatment: A New Era of Medicine
The implications of these discoveries extend far beyond diagnosis. By understanding how genetic variations affect specific brain regions, researchers can design treatments that address the root causes of structural abnormalities. This approach holds immense promise not only for managing existing conditions but also for preventing them altogether.
as the field of genetics continues to advance, the potential for personalized medicine grows exponentially. With a deeper understanding of how genes shape brain health,we are moving closer to a future where treatments are as unique as the individuals receiving them. This isn’t just science—it’s a transformative shift in how we approach healthcare.
Looking Ahead: The future of Brain Health
The integration of diverse datasets and cutting-edge technologies like single-cell RNA sequencing is revolutionizing our understanding of brain health. By combining these tools, researchers are uncovering the complex interplay between genetics and brain structure, offering new hope for millions of people affected by neurological and psychiatric conditions.
As we continue to explore the genetic underpinnings of brain health, the possibilities for innovation are endless. From targeted therapies to preventive measures, the future of medicine is being shaped by these groundbreaking discoveries. And with each new insight, we move one step closer to a world where brain health is understood, managed, and optimized like never before.
Unlocking the Genetic Secrets of the Brain: A New Era for Personalized Medicine
Recent breakthroughs in neuroscience are shedding light on the intricate genetic architecture of subcortical brain regions, areas critical for learning, memory, motor skills, and emotional regulation. These discoveries are not just academic—they hold the potential to revolutionize how we approach neurological and psychiatric disorders, paving the way for more personalized and effective treatments.
genetic Associations in Subcortical Brain Regions
One of the most striking findings from recent research is the identification of significant genetic associations within subcortical brain regions. The brainstem, in particular, has emerged as a hotspot for autonomous genetic links. Key genes such as CRHR1, MAPT, and ZNF786 have been linked to variations in subcortical brain volumes. These genes play a role in intracellular signaling and processes tied to brain aging, including tau pathology and vascular resistance.
Advanced Tools and Techniques Driving Discovery
To uncover these genetic connections,researchers employed cutting-edge tools like MAGMA and transcriptome-wide association studies (TWAS). These methods revealed that genes such as FOXO3 influence multiple brain structures, while members of the WNT family are tied to volumes in the brainstem and ventral diencephalon. Such findings are not just academic—they point to potential therapeutic targets for a range of brain disorders.
Integrating Single-Cell RNA Sequencing with GWAS
Another groundbreaking aspect of this research is the integration of single-cell RNA sequencing with genome-wide association studies (GWAS). This approach has allowed scientists to pinpoint specific cell types,such as dopaminergic neurons and astrocyte-like cells,as key contributors to brain volume variation. By understanding how genetic differences translate into structural changes during early brain development, researchers are gaining unprecedented insights into brain health and disease.
Implications for Neuropsychiatric and Neurological Disorders
The genetic foundations of subcortical brain volumes are deeply intertwined with conditions like Parkinson’s disease, ADHD, and other developmental or psychiatric disorders. Such as,the basal ganglia—a region crucial for motor control—has shown associations with genetic variants linked to Parkinson’s disease. These findings underscore the potential for genetic insights to inform targeted interventions for brain disorders.
Future Research Directions
This research opens exciting new avenues for understanding the genetic basis of brain structure and function. The integration of single-cell RNA sequencing with GWAS offers a powerful framework for future studies, enabling a more nuanced understanding of how genetic variations influence brain health. As one researcher noted, “This approach provides a roadmap for developing targeted therapies that address the root causes of neurological and psychiatric conditions.”
The Interplay Between Genetics and Lifestyle
while genetics play a significant role in shaping brain volume, lifestyle factors such as diet, physical activity, and environmental exposures also have a profound impact. This interplay suggests that daily choices can influence how genetic predispositions manifest, offering hope that proactive lifestyle changes may help mitigate risks for certain brain disorders. As the research highlights, “Genetics may load the gun, but lifestyle pulls the trigger.”
Conclusion
The discoveries surrounding the genetic architecture of subcortical brain regions mark a significant step forward in neuroscience. By uncovering the genetic underpinnings of brain structure and function, researchers are laying the groundwork for more personalized approaches to treating neurological and psychiatric disorders. as we continue to explore the interplay between genetics and lifestyle, the potential for improving brain health and quality of life for millions around the globe becomes increasingly tangible.
Unlocking the Secrets of Brain Volume: The Role of Genetic Research
Recent advancements in genetic research have shed light on the intricate relationship between brain structure and function. While these findings are undeniably groundbreaking, they remain correlational, highlighting the need for further investigation to establish definitive causal relationships. By combining single-cell RNA sequencing with Genome-Wide Association Studies (GWAS), researchers have pinpointed specific cell types that play a crucial role in brain volume variations. This discovery opens up exciting possibilities for developing targeted therapies to address neurological and psychiatric conditions.
Why This Research Matters
The study emphasizes the profound impact of genetic research on our understanding of the brain. By identifying the genetic factors that influence brain volume, scientists are paving the way for innovative treatments and preventive measures for conditions such as Alzheimer’s, schizophrenia, and depression. As one researcher noted, “The integration of single-cell RNA sequencing with GWAS data has identified specific cell types as significant contributors to brain volume variations, offering promising avenues for targeted therapeutic strategies.”
what’s Next in Brain Research?
While the current findings are a significant step forward, they also underscore the complexity of the human brain. future studies will need to delve deeper into the causal mechanisms behind these correlations. This could involve exploring how environmental factors interact with genetic predispositions or investigating the role of epigenetics in brain development. The ultimate goal is to translate these insights into actionable treatments that improve the lives of individuals affected by neurological and psychiatric disorders.
Key Takeaways
- Correlational Insights: Current findings highlight correlations between genetic factors and brain volume, but more research is needed to establish causation.
- Targeted Therapies: The identification of specific cell types offers hope for developing precise treatments for neurological conditions.
- Future Directions: Ongoing research will focus on understanding the interplay between genetics, environment, and brain health.
As we continue to unravel the mysteries of the brain, one thing is clear: genetic research holds the key to unlocking new frontiers in neuroscience.By building on these discoveries, we can move closer to a future where neurological and psychiatric conditions are not only treatable but preventable.
How do genetic variations identified in genes like CRHR1, MAPT, and ZNF786 contribute to variations in subcortical brain volumes?
Research have unveiled critical insights into the factors influencing brain volume, a key indicator of brain health. by leveraging large-scale genetic datasets and cutting-edge technologies, scientists are now able to identify specific genes and pathways that play a role in brain development, aging, and disease. These findings are not only deepening our understanding of the brain but also opening new avenues for personalized medicine and targeted therapies for neurological and psychiatric conditions.
The Genetic Blueprint of Brain Volume
Brain volume is a complex trait influenced by a combination of genetic and environmental factors. Recent studies have identified numerous genetic variants associated with variations in brain volume, particularly in subcortical regions such as the hippocampus, amygdala, and basal ganglia. These regions are crucial for functions like memory, emotion regulation, and motor control, making them key areas of interest in the study of brain health.
One of the most notable findings is the identification of genes like CRHR1, MAPT, and ZNF786, which are linked to variations in subcortical brain volumes. These genes are involved in processes such as intracellular signaling, tau pathology, and vascular resistance—factors that are closely tied to brain aging and neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Cutting-Edge Tools Driving Discovery
To uncover these genetic associations, researchers have employed advanced tools such as MAGMA (Multi-marker Analysis of GenoMic Annotation) and transcriptome-wide association studies (TWAS). These methods allow scientists to analyze the relationship between genetic variants and gene expression across different brain regions. For example, genes like FOXO3 and members of the WNT family have been found to influence multiple brain structures, providing potential targets for therapeutic interventions.
Another groundbreaking approach is the integration of single-cell RNA sequencing with genome-wide association studies (GWAS). This combination enables researchers to identify specific cell types, such as dopaminergic neurons and astrocyte-like cells, that contribute to variations in brain volume. By understanding how genetic differences affect these cells during early brain development,scientists can gain insights into the origins of brain disorders and develop targeted treatments.
Implications for Brain Disorders
The genetic foundations of brain volume are deeply intertwined with a range of neuropsychiatric and neurological disorders. For instance, the basal ganglia—a region involved in motor control—has shown associations with genetic variants linked to Parkinson’s disease. Similarly,variations in the hippocampus,a region critical for memory,have been linked to conditions like Alzheimer’s disease and depression.
These findings highlight the potential for genetic research to inform the development of targeted therapies for brain disorders. By understanding the specific genetic pathways involved, researchers can design interventions that address the root causes of these conditions, rather than just managing symptoms.
The Role of Lifestyle in Brain Health
While genetics play a significant role in shaping brain volume, lifestyle factors such as diet, physical activity, and environmental exposures also have a profound impact. Research suggests that daily choices can influence how genetic predispositions manifest,offering hope that proactive lifestyle changes may help mitigate risks for certain brain disorders. For example, regular exercise and a healthy diet have been shown to promote brain health and reduce the risk of neurodegenerative diseases.
As one researcher aptly put it, “genetics may load the gun, but lifestyle pulls the trigger.” This interplay between genetics and lifestyle underscores the importance of a holistic approach to brain health, combining genetic insights with lifestyle interventions to optimize outcomes.
Future Directions in brain Research
The integration of diverse datasets and advanced technologies is revolutionizing our understanding of brain health. By combining genetic data with facts on brain structure and function, researchers are uncovering the complex interplay between genes, cells, and environmental factors. This approach is paving the way for more personalized and effective treatments for brain disorders.
Looking ahead, future research will likely focus on further unraveling the genetic and cellular mechanisms underlying brain volume variations. This includes exploring how genetic differences influence brain development and aging, as well as identifying new therapeutic targets for neurological and psychiatric conditions. additionally, the role of epigenetics—the study of how environmental factors influence gene expression—will be a key area of investigation.
Conclusion
The discoveries surrounding the genetic architecture of brain volume represent a significant leap forward in neuroscience.By uncovering the genetic underpinnings of brain structure and function, researchers are laying the groundwork for more personalized approaches to treating neurological and psychiatric disorders. As we continue to explore the interplay between genetics, lifestyle, and brain health, the potential for improving quality of life for millions around the globe becomes increasingly tangible. This is not just science—it’s a transformative shift in how we understand and approach brain health.