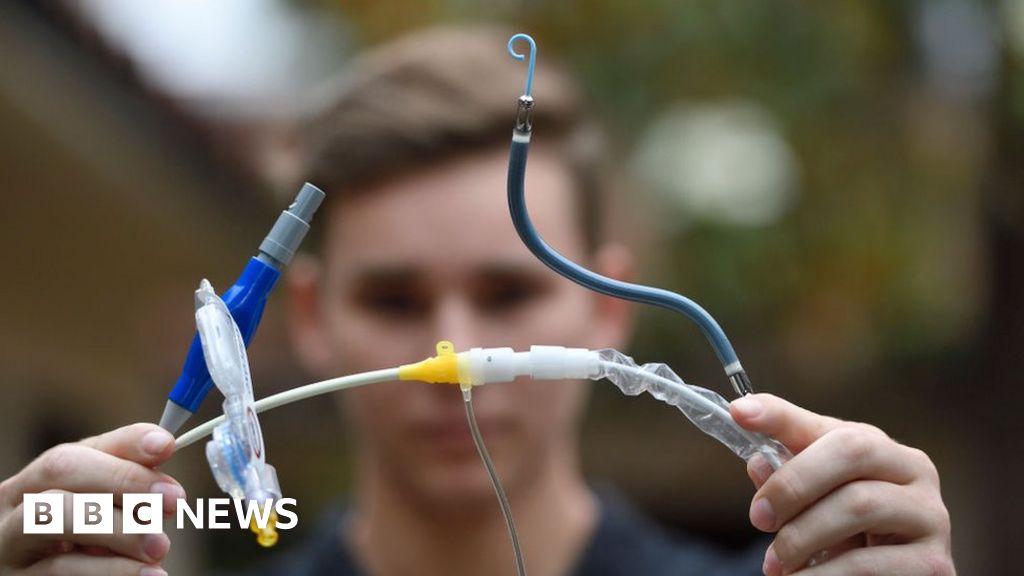
The US Food and Drug Administration (FDA) has issued a high-level alert regarding a heart pump that has been linked to numerous deaths and injuries. The Impella left sided pumps are used to provide temporary support to a patient’s heart during high-risk procedures or following a severe heart attack. However, the FDA has warned that incorrect usage of the device might result in the puncturing of the wall in the heart’s left ventricle.
This latest safety concern has prompted the device’s manufacturer, Abiomed, to provide new instructions for the pump. The FDA has classified this particular recall as the “most serious type” due to the potential for serious injuries or even death if the device is not used correctly. In addition to the risk of heart perforation, the FDA has also highlighted other adverse health consequences associated with the use of affected pumps, including hypertension, lack of blood flow, and fatalities.
It is important to note that this recall is categorized as a correction rather than a product removal, indicating that the device will remain on the market. The FDA’s notice relates to 66,390 devices that were distributed in the US over a two-year period starting from October 10, 2021. Despite the recall, Johnson & Johnson, which acquired Abiomed in 2022, has confirmed that the Impella heart pumps will continue to be available for patients.
The Impella heart pump, approved by the FDA in 2008, is inserted into the blood vessels and threaded through to the left ventricle, a crucial chamber responsible for pumping oxygenated blood throughout the body. However, Abiomed failed to fully disclose the risk of heart perforation during the pump’s insertion in a technical bulletin shared in October 2021, neglecting to inform the FDA at the time.
As a result, the FDA conducted an inspection of Abiomed’s office in Massachusetts in early 2023 and subsequently issued a warning letter to the company in September. The FDA criticized Abiomed for its failure to update them on the risk of heart perforation. The warning letter led to Abiomed issuing an “Urgent Medical Device Correction letter” in late 2023, containing revised instructions for the proper usage of the heart pump.
The implications of this safety concern and recall are significant for both the medical industry and patient safety. The FDA’s alert highlights the importance of proper communication between medical device manufacturers and regulatory bodies to ensure timely and accurate information sharing. It also underscores the need for continuous monitoring and evaluation of medical devices throughout their lifecycle.
In an era of rapid technological advancements in the healthcare sector, incidents like this remind us of the crucial role played by regulatory bodies in ensuring patient safety. This incident may prompt regulatory authorities to further tighten regulations and oversight processes to prevent similar occurrences in the future.
Furthermore, this incident serves as a reminder for healthcare providers to remain vigilant and strictly adhere to the usage guidelines provided by device manufacturers. Clear communication channels between manufacturers, regulatory authorities, and healthcare professionals are key to minimizing the risks associated with medical devices.
Looking ahead, the FDA is likely to increase its scrutiny of medical devices, particularly those used in critical procedures or with potentially severe consequences if misused. Stricter regulations and more frequent inspections can be anticipated to ensure the highest level of patient safety.
In conclusion, the FDA’s alert regarding the Impella heart pump serves as a wake-up call for the medical industry, highlighting the need for more stringent oversight and improved communication between manufacturers and regulatory bodies. As technology continues to advance, it is crucial that patient safety remains the top priority in the development, distribution, and usage of medical devices.