On March 13, the reporter fromBGIIt was learned that the State Food and Drug Administration issued a notice recently, approvingBGINovel Coronavirus (2019-nCoV) Antigen Detection Kit (Fluorescence Immunochromatography)medical instrumentsChanges to the registration certificate.this timemedical instrumentsChanges to the registration certificate have been addedBGIThe scope of application of new crown antigen detection products is applicable to professional medical institutions such as disease control and primary hospitals, or people in quarantine areas/sealed control areas who are “willing to be tested and tested”, which means that certain groups of people can purchase BGI’s new crown antigen products for self-testing. It is helpful to meet the diversified detection scenarios of overall epidemic prevention and control.
BGI’s new crown antigen detection product.
Antigen detection is a direct detection of the unique protein (ie antigen) in the virus, which can quickly detect positive cases in the acute infection period. Optimizing new coronavirus detection strategies and overall epidemic prevention and control plays an important role.
It is understood that BGI’s new coronavirus (2019-nCoV) antigen detection kit (fluorescence immunochromatography) adopts immunochromatography sandwich method, combined with fluorescent microspheres as tracer markers, to detect the oral cavity of people suspected of new coronavirus infection. The new coronavirus N antigen in throat swab and nasopharyngeal swab samples was tested.
BGI said that the test kit product needs to be used with a dry fluorescence immunoassay analyzer. The test results have high stability and good specificity, which can realize rapid detection, and have the ability to automatically interpret test results and manage test samples in information technology. The advantages.
According to BGI, the detection kit is regarding 10 times more sensitive than the traditional colloidal gold chromatography detection technology, and the results are automatically interpreted by a dry-type fluorescence immunoassay analyzer, which can achieve zero manual error. In addition, the product can be connected to the hospital Lis system (hospital inspection management system) to realize information management of test samples.
In terms of the dry-type fluorescence immunoassay analyzer equipped with the product, the instrument is small in size, easy to carry, and suitable for various detection scenarios such as customs, airports, schools, hospitals, cruise ships, clinics, township health centers, enterprise units, and CDCs; Equipped with a 4.3-inch high-sensitivity touch LCD screen, a reagent strip socket, support for Bluetooth or wireless network data transmission test results, information management of all test specimen information, traceable information; 15 minutes of rapid detection can be achieved, and multiple consecutive For rapid sample testing, one instrument can read at least 60-100 sample results in one hour.
BGI said that the product is easy to operate, fast to detect, does not require a specific laboratory environment, and does not require complex testing equipment, and is especially suitable for rapid population screening and triage management.
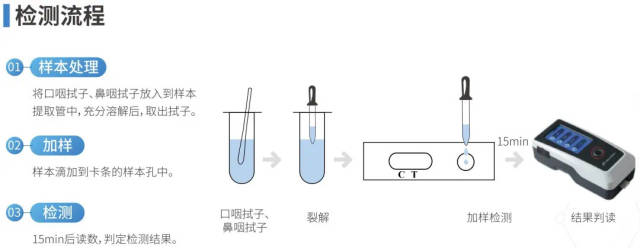
BGI’s new crown antigen detection product testing process.
In terms of the applicable population, BGI introduced that the first is those who have gone to primary medical and health institutions and have symptoms such as respiratory tract and fever and have symptoms within 5 days; Persons in entry quarantine, quarantine and control areas, and control areas; third, community residents who need antigen self-testing.
BGI also reminded that if primary medical institutions do not have nucleic acid detection capabilities, they can conduct antigen testing, and do a good job in training medical staff and communicating with patients. Isolation observers and community residents should carefully read the instructions and standardize their operations when conducting antigen tests. Once the antigen test is positive, it should be reported to the relevant departments immediately, and a nucleic acid test will be carried out to confirm.
(Article source: Southern Plus)



