A domestic biopharmaceutical company is going to the American Association for Cancer Research annual conference (AACR 2023) to publicize the clinical data of new anti-cancer drugs being developed on the international stage.
As AACR is considered the world’s largest international academic event in the field of oncology, we are looking at plans for technology export through the disclosure of clinical data for new anti-cancer drugs.
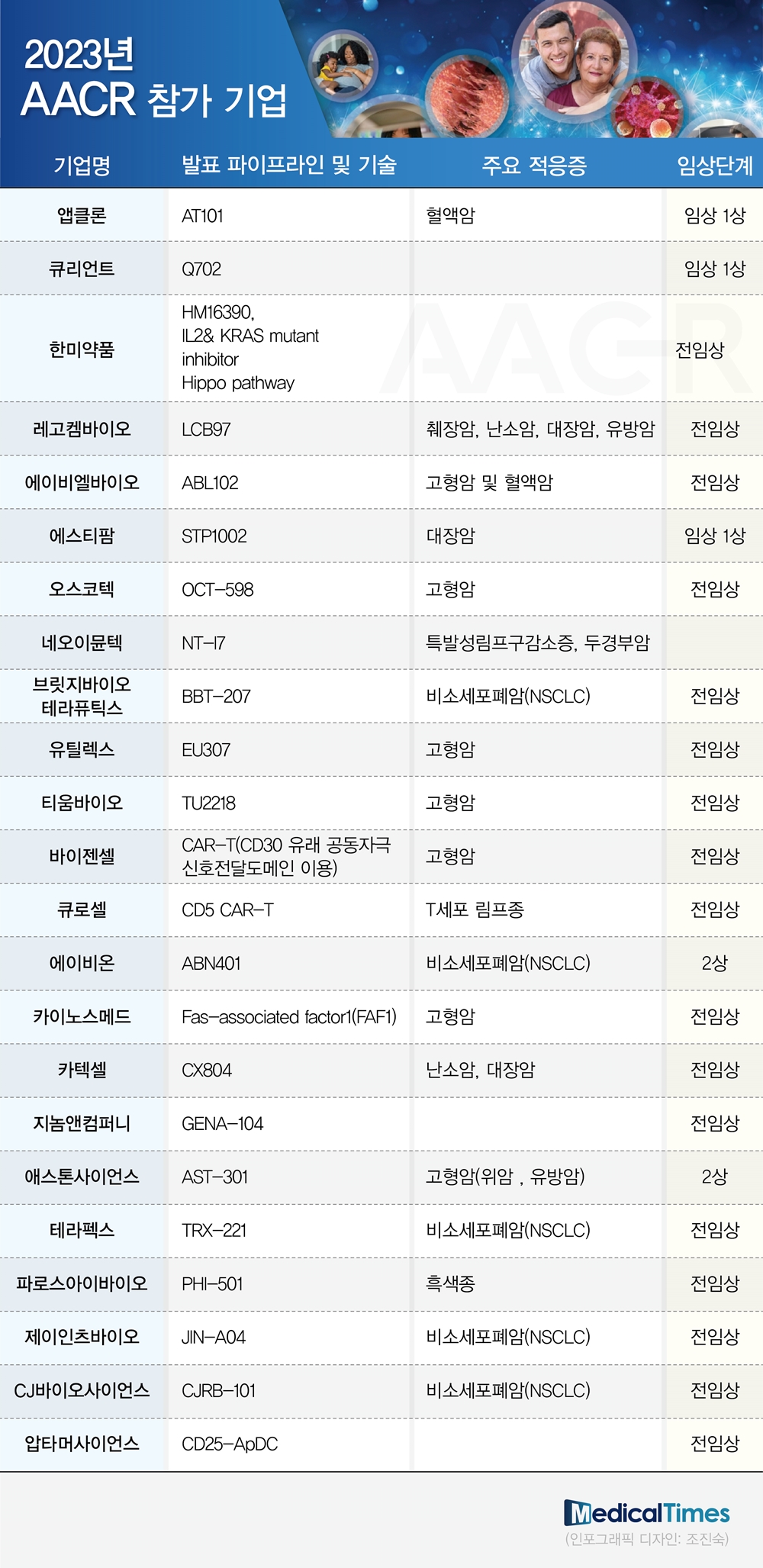
According to the pharmaceutical industry on the 14th, various companies ranging from traditional domestic pharmaceutical companies to emerging bio companies announced the announcement of clinical research results of new anti-cancer drugs that have been promoted so far through AACR.
AACR, which celebrates its 116th anniversary this year, has 48,000 members from 127 countries and is the largest international academic event in the oncology field along with the American Society of Clinical Oncology (ASCO).
AACR (American Association for Cancer Research) 2023 will be held for six days from April 14th to 19th in Orlando, USA.
Korea and the U.S. release anticancer drug candidate research following last year
At this AACR, traditional pharmaceutical companies that have taken the lead in developing new anti-cancer drugs in Korea will make presentations. Hanmi Pharmaceutical plans to disclose seven research results, the largest among domestic companies.
Specifically, interleukin-2 (IL-2) immuno-anticancer drugs, KRAS mutation-targeted SOS1 inhibitors, Hippo signaling pathway target novel YAP/TAZ-TEAD inhibitors for solid cancer, etc. A poster presentation regarding the possibility of treatment, etc.
HM16390, which is being developed by Hanmi Pharmaceutical, is a candidate material that has improved safety as well as strong antitumor efficacy by optimizing the binding force between IL-2 receptors. It is a formulation that can be administered subcutaneously once per anticancer cycle, and toxicity studies are currently underway to enter phase 1 clinical trials.
In addition, on the 19th, we plan to announce the results of a study that confirmed the strong anticancer effect of HM97662, an EZH1/2 double inhibitor. HM97662 is being developed as a treatment for advanced solid cancer, and this year, it presents the possibility as a treatment for T-cell lymphoma in terms of indication expansion.
An official from Hanmi Pharm said, “Hanmi’s AACR announcement this year can be explained with the keywords ‘innovation’, ‘expansion’, and ‘new modality’. “We will continue to discover new innovative programs while further advancing existing R&D tasks.” said.
LegoChem Bio and ABL Bio, which drew attention as a big deal last year, will also announce the contents of the LCB97 and ABL102 pipelines, respectively.
LegoChem Bio, which has ADC technology that combines anticancer drugs with antibodies targeting cancer cells, plans to disclose the results of research on immuno-anticancer drugs using the low-molecular compound ‘LCB33’. The results of applying the tumor model and the toxicity evaluation results for monkeys are disclosed.
In addition, ABL Bio will present the preclinical data of ABL102 (ROR1x4-1BB), a candidate for immuno-oncology, as a poster.
ABL102 is the first ROR1x4-1BB bispecific antibody to which Grabody-T, a core platform technology of ABL Bio, is applied. Memory T-cells possessed by Grabody-T’s 4-1BB antibody are specialized in maintaining long-term effects and preventing recurrence, so treatment for carcinomas with high unmet needs is possible because resistance develops in a short period of time and the recurrence rate is high. It is hoped that this will be possible.
Domestic CAR-T leader next-generation candidate materials
One thing to note is that while clinical trials for CAR-T treatments are underway in Korea, announcements are being made by companies that are evaluated as leaders.
First, Curocell will present the research results of CD5 CAR-T, a treatment for T-cell lymphoma.
The CD5 CAR-T treatment to be announced this time is a CAR-T treatment that targets CD5 present on the surface of T cells. A new antibody discovered by Curocell and a technology that minimizes the phenomenon of fratricide, which has been pointed out as a problem with the existing CD5 CAR-T treatment, was applied.
In addition, AbClon plans to disclose some of the non-clinical and phase 1 clinical results for AT101, which completed US patent registration in November last year.
Unlike the four previously commercialized CD19 CAR T treatments, AbClon is expected to explain the difference in acting on a new region of the CD19 protein.
In addition, Baxel Bio will present the preclinical results of an anti-PD-L1 CAR treatment targeting solid cancer for which it has recently applied for a patent.
Baxel Bio’s anti-PD-L1 CAR treatment uses a newly developed scFv. In actual preclinical tests, it showed strong killing ability once morest cancer cells, but showed no toxicity, proving its efficacy and safety. In addition, due to the nature of the treatment, it can be applied to all cancer types expressing PD-L1, showing the possibility of development as a general-purpose treatment.
In addition, Onconic Therapeutics, a subsidiary of Jeil Pharmaceutical, will announce the results of two non-clinical studies on ‘Nesuparib’, a new drug candidate for a dual inhibitory target anti-cancer drug, and Tium Bio on ‘TU2218’, an immuno-anticancer drug.
▲Medpacto ▲Prestige Biopharma ▲NeoImmuneTech ▲Qurient ▲CJ Bioscience ▲Aptamer Science will also participate and show off their research results on anti-cancer treatments.






