It has been suggested that it can improve degenerative neurological diseases such as dementia by regulating the intestinal microflora.
A research team at Washington University School of Medicine in St. Louis, USA found that gut microbes affect the formation of tau protein, which is associated with Alzheimer’s disease, and recently published a related paper in the journal ‘Science’.
Tau protein, along with amyloid beta plaques, is a substance found in abundance in the brains of dementia patients. While a lot of research is being done on treatments to remove amyloid beta, there are claims that tau protein is more closely related to dementia.
On the other hand, the fact that the composition of the intestinal microflora of Alzheimer’s patients and healthy people is different from each other is known through recent studies. However, it was unclear whether this was a cause or a consequence of dementia and how changes in the gut microbiome affected the course of the disease.
The research team manipulated the genes of the experimental mice and expressed the human tau protein mutation to cause brain damage and cognitive decline similar to Alzheimer’s. When the mice were 9 months old, tau protein accumulated, resulting in cranial nerve damage and brain atrophy.
In addition, the research team also had mice with human APOE gene mutations. The APOE gene is known to be closely related to the onset of dementia. People with the APOE4 gene are three to four times more likely to develop dementia than people with the APOE3 mutation, which differs by only one or two amino acids.
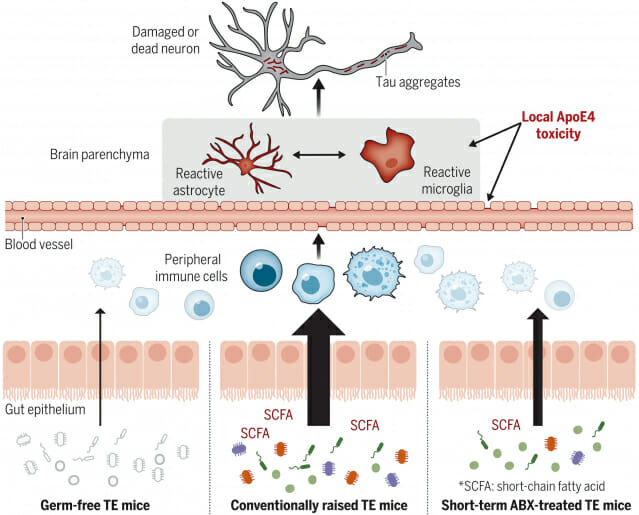
Right following birth, the genetically engineered mice were raised in an aseptic environment so that they did not have any intestinal microbes. At 40 weeks of age, these mice showed less brain damage than mice with normal gut microbes.
Genetically engineered mice raised in a normal environment had the same intestinal microflora as normal mice. Injecting antibiotics into these mice when they were two weeks old completely changed the composition of their gut microbiome. Male mice showed less brain damage at 40 weeks of age. In particular, the effect was greater in mice with the APOE3 mutation than in mice with the APEO4 mutation, which are vulnerable to dementia. On the other hand, antibiotic administration had little effect on cranial nerves in female rats.
In addition, the research team found that three types of short-chain fatty acids produced by metabolic activities of intestinal microbes affect brain nerve damage. The research team estimated that these fatty acid components activate immune cells in the bloodstream, which in turn activate immune cells in the brain through some pathway, causing damage to brain tissue.
When these three short-chain fatty acids were injected into middle-aged mice without intestinal microbes, the activity of immune cells in the brain increased, similar to what appears when tau protein accumulates.
related articles
The research team expects that this study will open the way to prevent and treat degenerative brain diseases by controlling intestinal microbes through antibiotics, probiotics, and dietary therapy. It is said that the possibility of delaying or treating dementia can be found by conducting intestinal microbiome treatment right before the symptoms of dementia appear in middle age.
“The most exciting thing is that regulating the gut microbiome can have an effect on the brain without injecting anything directly into it,” said David Holtzman, a professor at the University of Washington.



