2024-08-11 04:00:07
CNRS scientists have just synthesized copper-based compounds whose luminescence irreversibly changes color once a critical temperature is exceeded. This color change, a unique witness to the thermal experience of their environmentarouses interest in the development of innovative procedures for control non-destructive.
Recent events clearly highlight the dependence of modern societies on sources of supply of strategic raw materials. A crucial dependence is thus found in the field of luminescent materials used daily in screens (mobile phones, computers) as well as in many detection applications. Indeed, the properties of luminescence are very often generated by photoactive derivatives based onatoms heavy, precious and/or rare, sometimes presenting significant toxicities. A serious limitation for their use in large scale. In this context, luminescent materials based on Cu(I) ions are particularly attractive given their low cost and the natural abundance of copper. In addition, their flexible molecular structure, which can be easily modified by the application of external stimuli (temperature, pression…), allows the modulation of the characteristics of their luminescence.
Chemists from the Rennes Institute of Chemical Sciences (CNRS/Université Rennes/ENSCR/INSA Rennes) have developed a simple and rapid synthesis of a large family of metal complexes exhibiting remarkable photoluminescence properties at room temperature.
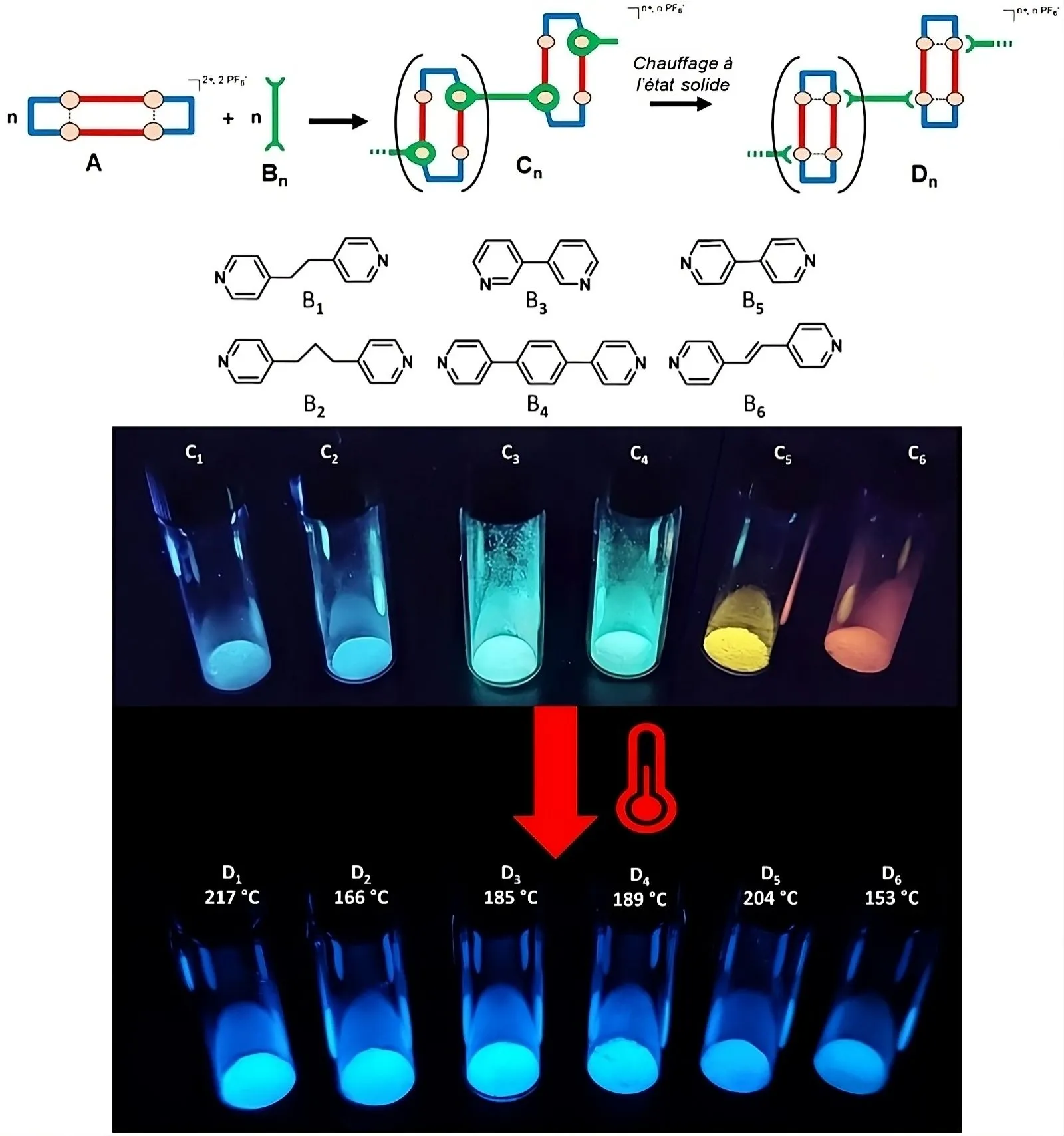
For this synthesis, the scientists imagined a luminescent precursor consisting of four Cu(I) ions assembled together by organic molecules whose nature allows the emission wavelength to be modulated. They then observed an irreversible change in the color of the emission of the material in the solid state above a temperature specific to each compound. They were able to attribute the origin of this unprecedented transition to a dissociation of the polymeric structure of these assemblies activated by crossing a limit temperature.
Such original behaviors are the direct consequence of the great flexibility of the assembly of luminescent precursors based on Cu(I) ions. It is indeed possible to dissociate these structures by increasing the temperature, while preserving the molecular structure of the precursor intact within the new material at the origin of the luminescence. New material which then presents a luminescence clearly different from that of the initial polymeric assembly.
A unique property that makes these materials witnesses to the thermal experience of their environment. We can therefore consider inserting them into technological devices where we want to ensure, for their proper functioning, that they have not exceeded certain temperatures. Results that are the subject of an article in the journal Adv. Opt. Mater.
Editor: CCdM
Reference:
A. Schlachter, F. Moutier, R. Utrera-Melero, J. Schiller, A. Moustafa Khalil, G. Calvez, M. Scheer, K. Costuas & C. Lescop.
Photoluminescent Cu(I) Assemblies With High Temperature Solid-State Transitions as a New Class of Thermic History Tracers
Adv. Opt. Mater, 2024
1723413366
#compounds #irreversibly #change #color #exceeding #temperature