The latest research results of power batteries are exposed:It takes only 18 seconds to fully charge.
The cost is only one-sixth of the mainstream lithium-ion battery, and the paper has been published in the journal Nature.
This kind of battery can be used normally at low temperature and stable at high temperature.
One of the professors responsible for this patented technology has established a battery company and obtained the patent authorization. The company has raised $8.1 million to date.
The remaining question: Will this fast-charging, lower-cost, and safer battery affect the current power battery pattern?
what kind of battery
According to the research results, the cost of this bidirectional, fast-charging aluminum-chalcogen battery is as low as $8.99 per kWh, which is 12%-16% of the current cost of lithium-ion batteries.
The expected battery energy density of this battery is 526Wh/I, which is comparable to the energy density of lithium-ion batteries such as graphite-NMC622.

At the same time, no additional active cooling system is required, thermal runaway and fire prevention can be avoided.
And there is almost no dendrite formation, which prevents the battery from short circuiting.
How did it happen?
First, raw materials for new batteries are abundant and cheap.

The new battery cathode is made of chalcogen elements, such as sulfur and selenium.
The negative electrode is aluminum, and the electrolyte is molten chloroaluminate composed of NaCl-KCl-AlCl3.
Aluminum is known to be the most abundant metallic element on earth. At the same time, elemental sulfur, NaCl, KCl, and AlCl3 are also common chemical substances.
And this study shows that even if low-quality aluminum such as aluminum foil for food packaging is used to make the negative electrode, it will not affect the performance of the battery.

These factors have greatly reduced battery costs.
Secondly, the alkali metal chloroaluminate melt used as the electrolyte has a much lower eutectic point than the general molten salt system, regarding 93°C.
This ensures that the battery can operate at temperatures as low as 110ºC, while remaining “self-heating” during use, maintaining its own temperature with proper insulation.
This means no active cooling system is required.
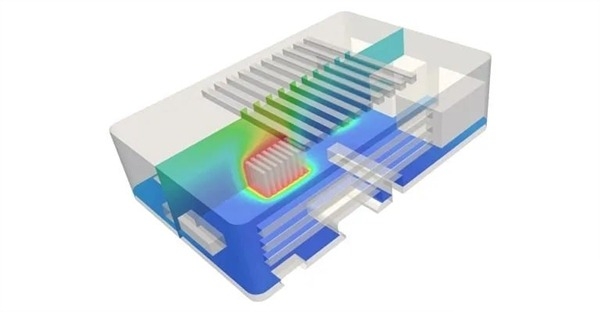
Due to thermal effects, general lithium-ion batteries require a cooling system to keep the battery at an optimal operating temperature and improve efficiency.
At the same time, even if the temperature exceeds 500°C, the molten salt electrolyte is still thermally stable and non-volatile, and will not vaporize at high temperatures, causing the battery to explode or catch fire.
Moreover, molten salt electrolyte has another advantage besides low melting point:Prevent battery short circuit.
This is because Al3+ in the electrolyte has the characteristics of desolvation, which can prevent the formation of aluminum dendrites.
And most importantly, this new type of battery has good cycle stability, so it can be charged quickly.
How is this possible?
Research Principle
The paper mainly demonstrates the performance of aluminum selenium battery and aluminum sulfur battery.
Experiments have shown that in NaCl-AlCl3 electrolyte (melting point regarding 115°C), the discharge reaction of AlSe battery is very stable even at 180°C, with an average voltage of regarding 0.88V.
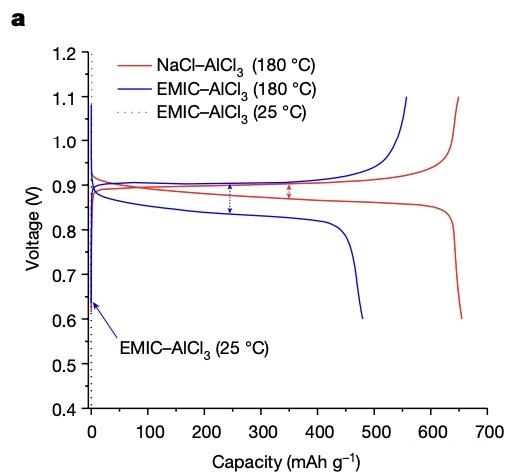
At the same time, when the discharge time is shortened to 5 hours or fully charged in 2 hours, the Al-Se battery has no voltage decay following 50 cycles, and the battery capacity can be maintained at 300mAh/g.
When the full charge time is shortened to 18 seconds, the capacity of the Al-Se battery remains at 75mAh/g.
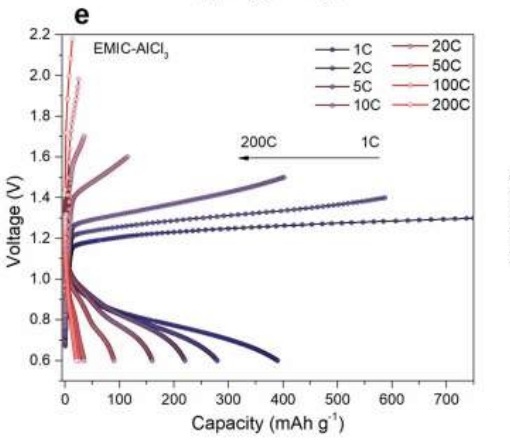
As a comparison, for general aluminum metal electrodes, the electrolyte uses EMIC–AlCl3 (EMIC: 1-ethyl-3-methylimidazolium chloride).
However, when the full charge time of the EMIC-AlCl3 battery is shortened to 6 minutes (10C) or below, the battery capacity is already close to zero.
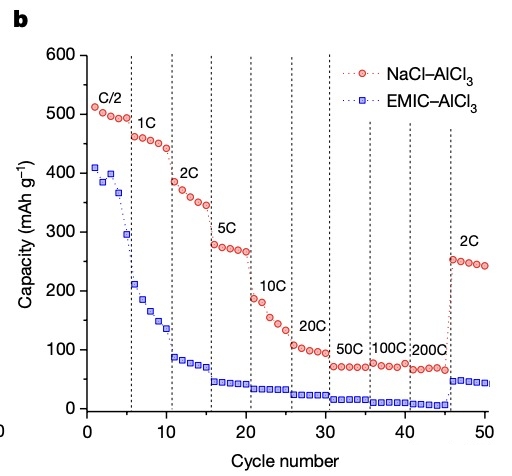
The researchers also changed the charging rate and found that the reversible capacity of the Al-Se battery was 520mAh/g when the full charge time was 2 hours (C/2); 190mAh/g in 6 minutes; 75mAh/g in 18 seconds .
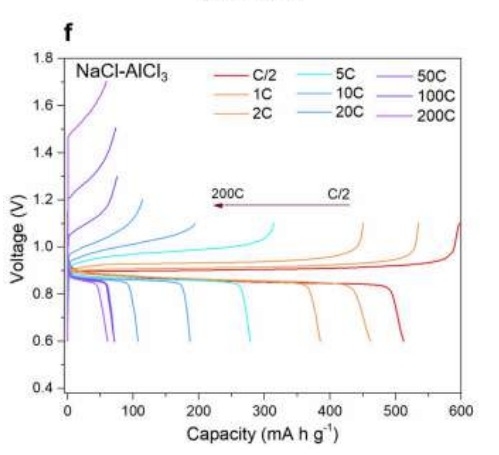
For aluminum-sulfur batteries, in NaCl–KCl–AlCl3 electrolyte (eutectic temperature regarding 93°C), the operating temperature can reach 110°C, and the battery capacity is 525mAh/g.
When the discharge time is constant at 2 hours, even if the charging rate of the aluminum-sulfur battery is increased and the full charge time is shortened to 6 minutes, the battery capacity can still be maintained at 500mAh/g.
When the full charge time is shortened to 18 seconds, the capacity of the aluminum-sulfur battery remains at 210mAh/g.
In contrast, the full charge time was shortened to 72 seconds with the EMIC–AlCl3 battery, and the battery capacity was close to zero following the temperature increased.
Moreover, under the cycle scheme set in the paper, the aluminum-sulfur battery can maintain hundreds of cycles at a high charge rate (full charge time is 6-12 minutes) and an ultra-high charge rate (full charge time is 36-72 seconds).
At the same time, the deposition of Al3+ ions in the molten salt electrolyte has a kinetic advantage, so this new type of battery can be charged more easily than discharged to achieve higher performance.
research team
Donald Sadoway (Sadoway) is a professor in the Department of Materials Science and Engineering at the Massachusetts Institute of Technology. His research focuses on electrochemistry in non-aqueous media, including molten salts, physical chemistry and electrochemistry of low-temperature electrolytes.
In 2020, Sadoway received seed funding from the MIT Energy Initiative. In 2022, Sadoway won the European Inventor Award for his liquid metal battery.
And, Sadoway and Luis Ortiz co-founded Avanti, a battery startup, with Sadoway serving as chief scientific advisor. The company has been granted a patent for this aluminum-chalcogen battery thesis research. In April last year, the company completed Series A financing with a financing amount of US$8.1 million.
Investment companies include Bill Gates’ Breakthrough Energy, and Eni Next, the venture capital subsidiary of Eni Group (one of the world’s seven largest oil conglomerates).

Sadoway said the company’s priority now is to demonstrate that the aluminum-chalcogenide battery can operate at scale, followed by a series of stress tests, including running hundreds of charge cycles.
In addition to being used as a power battery, smaller-scale aluminum-chalcogenide batteries can also be used for electric vehicle charging piles, reducing construction costs and increasing charging speed.

And this kind of battery can also power a single household or small and medium-sized business, with a storage capacity of regarding tens of kilowatt-hours.
Sadoway said the point of the research paper is to remind people: “If you are willing to invest time and money, there are better, cheaper and safer technologies than lithium-ion batteries that can be researched.”
Link to the original text of the Nature paper:https://www.nature.com/articles/s41586-022-04983-9



