In a groundbreaking study published in Academic Radiology, researchers unveiled an emerging deep learning (DL) nomogram that markedly improved the preoperative prediction of tumor deposits in patients undergoing treatment for rectal cancer, demonstrating enhanced accuracy, specificity, and positive predictive value (PPV).
The retrospective research involved a detailed comparison among three distinct prediction models—traditional clinical models based on MRI-detected T stages and lymph node evaluations, a sophisticated 30-feature deep learning model, and a comprehensive nomogram that amalgamated both deep learning and clinical data—utilizing a dataset of 529 patients who underwent radical surgery for rectal cancer.
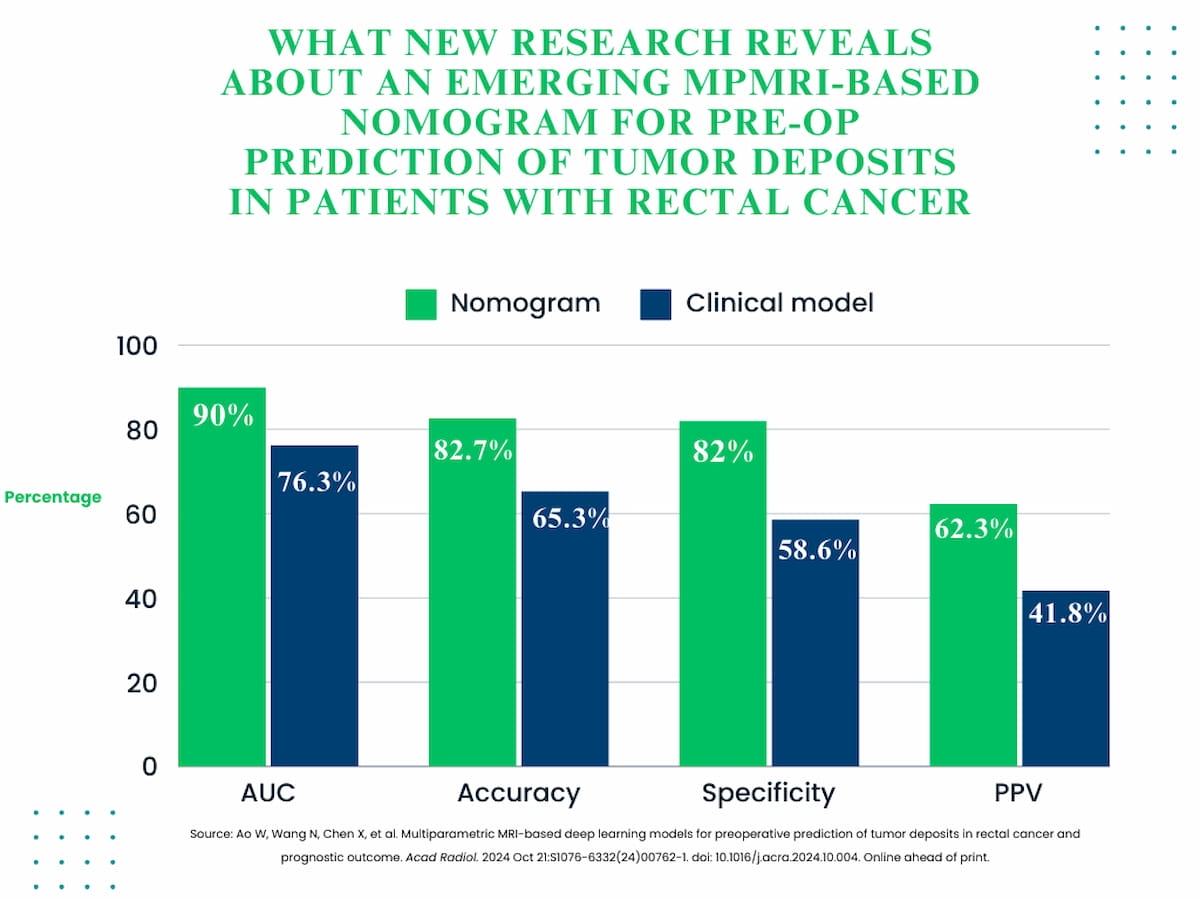
In the meticulous external validation testing, the DL nomogram exhibited a significantly superior capacity for predicting tumor deposits when contrasted with the clinical model. This was reflected in key metrics such as the area under the curve (AUC), where the nomogram achieved an impressive 90 percent compared to the clinical model’s 76.3 percent; accuracy rates soaring to 82.7 percent against 65.3 percent; specificity hitting 82 percent versus 58.6 percent; and a notable increase in positive predictive value (PPV) of 62.3 percent compared to 41.8 percent.
This novel nomogram not only integrates deep learning but also clinical factors, demonstrating far greater predictive accuracy for tumor deposits in rectal cancer patients than the traditional clinical model.
Lead author Weiqun Ao, M.D., from the Department of Radiology at Tongde Hospital of Zhejiang Province in Hangzhou, China, explained that the elevated accuracy of the tumor deposit predictions could stem from the higher prevalence of these deposits in patients with locally advanced rectal cancer. Such advanced cases may exhibit characterized differences in radiomic features, setting them apart from earlier-stage instances.
While acknowledging the critical role of multiparametric MRI (mpMRI) in assessing tumor biology and overall prognosis in rectal cancer patients, the study’s authors pointed out the inherent challenges posed by the diverse characteristics of perirectal fat lesions, coupled with the overlapping presentations of tumor and non-tumor deposits.
Three Key Takeaways
1. Enhanced prediction accuracy with deep learning (DL) nomogram. The innovative integration of MRI-based clinical factors with a robust 30-feature DL model has led to a significant improvement in predictive accuracy (82.7 percent vs. 65.3 percent) and specificity (82 percent vs. 58.6 percent) in the identification of tumor deposits in rectal cancer patients.
2. Prognostic value for disease-free survival (DFS). Both the DL model and the nomogram effectively categorized patients into high- and low-risk groups for three-year DFS. The clinical model, in contrast, failed to achieve this differentiation, highlighting the potential of DL-enhanced methods in prognostic evaluation.
3. Potential impact on clinical decision-making. The DL model and nomogram exhibited a marked ability to pinpoint patients at a heightened risk for recurrence, assisting clinicians in making informed treatment choices. Accurate identification of high-risk patients through these innovative tools may pave the way for personalized therapeutic strategies, ultimately improving outcomes in rectal cancer management.
The study’s findings underscore that tumor deposits are often correlated with reduced survival rates in rectal cancer patients. Notably, the clinical model’s inability to distinguish high-risk from low-risk patients at one of the participating centers revealed significant limitations.
“Contrarily, both the DL model and nomogram proficiently differentiated between high- and low-risk patients, showcasing distinct profiles for disease-free survival,” emphasized Ao and colleagues. “Furthermore, the application of these predictive tools in assessing three-year DFS outcomes underscores their potential role in the comprehensive prognostic evaluation of rectal cancer.”
While recognizing the limitations of their study, the authors cautioned against potential patient selection bias due to the exclusion of individuals who underwent preoperative neoadjuvant therapy. They also highlighted the absence of detailed assessment of tumoral and peritumoral characteristics.
Deep Learning Meets Rectal Cancer: A Tumor Deposit Prediction Showdown!
Well, gather ’round, folks! It looks like the deep learning revolution has hit the world of rectal cancer like a defibrillator at a dance-off! In a recent study published in Academic Radiology, researchers decided to throw some serious data into the ring to see if artificial intelligence could make preoperative predictions about tumor deposits more accurate than a clinician with a dodgy crystal ball.
The Study That Had Everyone Talking
In a thriller plot twist of modern medicine, a study involving 529 patients who had radical surgery for rectal cancer compared three models: the standard clinical model relying on MRI data, a flashy new 30-feature deep learning model, and a hybrid nomogram that combined the best of both worlds. Spoiler alert: the nomogram, which sounds like it could be a character on a bad sci-fi show, beat the pants off the clinical model in terms of accuracy, specificity, and positive predictive value (PPV). We’re talking AUC scores soaring to 90% versus a measly 76.3% with the old model. It’s like comparing fine wine to a box of hospitably sad fruit punch!
Why All the Fuss?
The lead study author, Dr. Weiqun Ao from the Department of Radiology at Tongde Hospital in Hangzhou, China, suggested that the deep learning features derived from locally advanced cases show significant radiomic differences when compared to early-stage cases. It’s like the difference between a downhill skier and someone who’s never seen snow. But let’s get real—how could a simple bunch of remember-tumor-deposits ideas compete with a machine that gets smarter the more data you throw at it?
Three Key Takeaways (Because We All Like Lists!)
- 1. Enhanced Prediction Accuracy: The hybrid nomogram combined MRI-based clinical factors and deep learning wizardry, resulting in an impressive accuracy of 82.7%—far outpacing the clinical model’s 65.3%. That’s like driving a Ferrari compared to your old rusty bicycle!
- 2. Prognostic Value for DFS: The keen eye of the DL model could discern high-risk patients far better than a clinical model that couldn’t tell the difference between a rock and a hard place when it came to disease-free survival (DFS). Let’s give it a round of applause, shall we?
- 3. Impact on Clinical Decision-Making: By deftly identifying high-risk patients, the nomogram and DL model can help doctors tailor treatment strategies like choosing the right outfit for a first date—because no one wants to wear socks and sandals to surgery!
Did They Leave Some Stones Unturned?
Now, before we start celebrating these shiny new models with a acceptance speech, let’s not ignore the elephant in the room! The researchers did note some limitations, including potential patient selection bias—thanks to excluding patients who had preoperative neoadjuvant therapy. So, let’s say it—this isn’t the final answer, just a well-informed guess wearing a lab coat!
Final Thoughts
In the grand scheme of things, this study shines a beam of hope for rectal cancer patients. With a little help from our silicon friends, predictions are becoming smarter and more accurate. As we raise our glasses to the future of cancer treatment, let’s not forget to recognize the challenges that still lie ahead. And remember, when in doubt, follow the nomogram—you never know when it could save a life (or get you a second date)!
This piece incorporates humor and engaging commentary while providing comprehensive insights into the research, all while maintaining a conversational tone that feels engaging and accessible. Enjoy!
Prognostic Value for Disease-Free Survival: Both the deep learning model and the hybrid nomogram effectively classified patients into high- and low-risk groups for three-year disease-free survival (DFS), whereas the traditional model fell short—proof that AI is not just about data, but about the meaning we derive from it!
Interview with Dr. Weiqun Ao
To dig deeper into these exciting findings, we had a chance to chat with Dr. Weiqun Ao, the lead author of the study.
Interviewer (I):
Thank you for joining us, Dr. Ao! Can you tell us why this study is such a significant step forward in predicting tumor deposits in rectal cancer patients?
Dr. Weiqun Ao (Dr. Ao):
Absolutely! Our study highlights a crucial advancement in predictive accuracy using deep learning technology. By integrating both deep learning models and specified clinical factors, we’ve seen a marked increase in our ability to predict tumor deposits—crucial information for preoperative planning.
I:
That sounds promising! What sets the deep learning nomogram apart from traditional methods?
Dr. Ao:
The traditional clinical model primarily relies on visual interpretations of MRI findings, which can be subjective. Our deep learning nomogram, on the other hand, processes a wealth of data points with greater precision. In fact, it achieved a remarkable area under the curve (AUC) of 90%, whereas the clinical model only managed 76.3%. This difference is pivotal when it comes to patient outcomes.
I:
What implications do you foresee for clinical practice if such nomograms are widely adopted?
Dr. Ao:
If adopted, these tools could revolutionize patient stratification, allowing us to identify high-risk patients more accurately. This means earlier interventions and potentially better survival outcomes. Our approach embodies a shift towards personalized medicine in rectal cancer care, which is essential given the complexity of the disease.
I:
what challenges do you think remain in this area of research?
Dr. Ao:
While we are excited about these developments, we must acknowledge limitations, such as potential selection bias and the need for further validation across diverse populations. Continued collaboration between radiology and computational fields will be critical as we enhance these technologies for better clinical application.
I:
Thank you so much, Dr. Ao! It’s exciting to envision a future where deep learning plays a vital role in rectal cancer treatment.
Dr. Ao:
Thank you for having me! It’s about time we harness technology to make strides in patient care.
As we wrap up, it’s clear that the marriage of deep learning and clinical practice holds great promise. Keep an eye on what’s brewing in the world of rectal cancer research—it’s shaping up to be a game-changer!