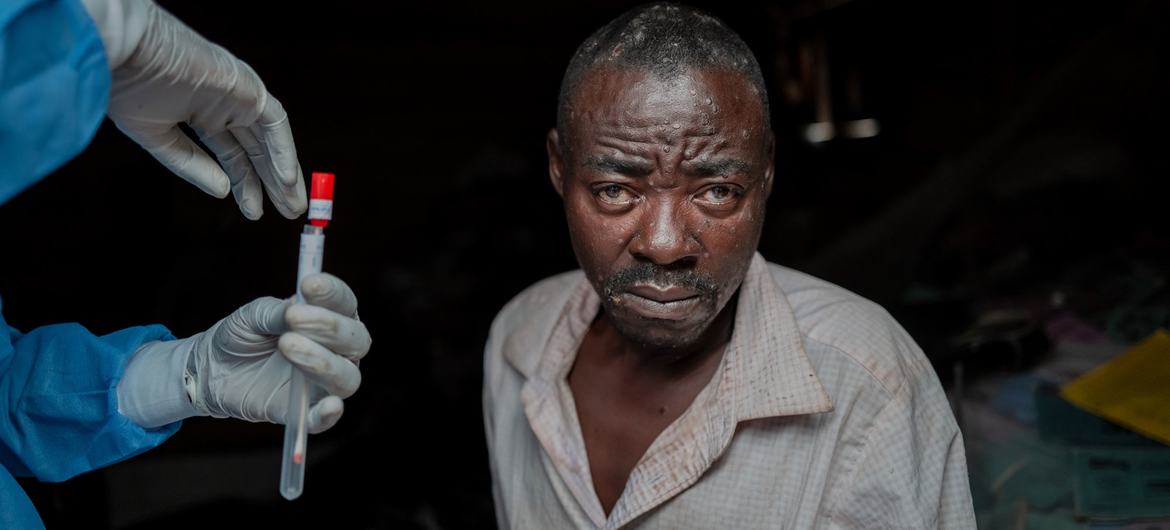
Ginebra.- The World Health Organization (WHO) approved the use of the first MPOX vaccine on Friday, with the aim of facilitating greater and timely access for millions of people at risk in Africa, where the latest outbreak has infected more than 20,000 people so far this year.
Danish pharmaceutical company Bavarian Nordic‘s MVA-BN vaccine was prequalified by the WHO on Friday and has already been approved in Europe and the United States for use in adults.
WHO approval will speed up access for millions of people, helping to reduce transmission and contain the outbreak.
WHO Director-General Tedros Adhanom Ghebreyesus said that prequalification of the vaccine is an important step in the fight against the mpox virus in Africa.
“We now urgently need to scale up procurement, donations and distribution to ensure equitable access to vaccines where they are most needed, along with other public health tools, to prevent infections, stop transmission and save lives,” Tedros said.
Since the start of the global mpox outbreak in 2022, some 120 countries have confirmed more than 103,000 cases.
The WHO prequalification is based on the evaluation of information submitted by the manufacturer, also reviewed by the European Medicines Agency, the regulatory agency of record for this vaccine.
Since the beginning of the global outbreak in 2022, the safety and efficacy of the vaccine has been tested in clinical studies and in the real world, in different contexts, mainly with the emergence of new strains of the virus.
The MVA-BN vaccine can be administered to people aged 18 years and older in two injectable doses four weeks apart. After prior cold storage, the vaccine can be stored at 2-8°C for up to eight weeks.
The health agency also recommends using a single dose in outbreak situations with supply constraints, and stresses the need to collect more data on the number of doses.
WHO reviewed all available evidence and recommended the use of MVA-BN vaccine in the context of an mpox outbreak for people at high risk of exposure.
Although MVA-BN is not currently licensed for use in persons under 18 years of age, this vaccine can be used “off-label” in infants, children and adolescents, and in pregnant and immunocompromised individuals. This means that the use of the vaccine is recommended in outbreak contexts where the benefits of vaccination outweigh the potential risks.
To date, data show that a single dose of MVA-BN vaccine administered prior to exposure is estimated to be 76% effective in protecting people against mpox, and the two-dose regimen is estimated to be 82% effective. Post-exposure vaccination is less effective than pre-exposure vaccination.
Since the WHO Director-General activated the emergency use listing for mpox vaccines on 7 August 2024, WHO has conducted programmatic and product capacity assessments.
“The findings of the assessments are particularly relevant in the context of the declaration of a public health emergency of international concern related to the resurgence of mpox in Africa,” said Dr Rogerio Gaspar, WHO Director of Regulatory and Prequalification.
Gaspar added that the health agency is advancing in the procedures for prequalification and inclusion in the list of vaccines for emergency use with the manufacturers of two other mpox vaccines. “We have also received so far six expressions of interest for mpox diagnostic products for inclusion in the emergency use list,” he added.
Once the vaccine is acquired, international agencies such as the United Nations Children’s Fund (UNICEF) and Gavi, the Vaccine Alliance, will be able to help distribute it globally, particularly to communities in Africa, such as the Democratic Republic of the Congo.
More than 120 countries have confirmed over 103,000 cases of mpox since 2022. In 2024 alone, there were 25,237 suspected and confirmed cases and 723 deaths from different outbreaks in 14 countries in Africa, as of September 8, 2024.
#approves #vaccine #monkeypox
2024-09-16 23:20:13
Interest from manufacturers for future vaccine development.
Table of Contents
Here is a comprehensive and SEO-optimized article on the topic of the WHO’s approval of the first MPOX vaccine:
WHO Approves First MPOX Vaccine, Paving Way for Global Access
In a crucial step forward in the fight against the ongoing MPOX outbreak, the World Health Organization (WHO) has approved the use of the first MPOX vaccine, developed by Danish pharmaceutical company Bavarian Nordic. This prequalification of the MVA-BN vaccine is expected to facilitate timely access to millions of people at risk in Africa, where the outbreak has infected over 20,000 people so far this year.
Accelerating Access to Vaccines
The WHO’s approval of the MVA-BN vaccine is expected to speed up access to vaccines for millions of people, helping to reduce transmission and contain the outbreak. The vaccine has already been approved in Europe and the United States for use in adults. According to WHO Director-General Tedros Adhanom Ghebreyesus, “We now urgently need to scale up procurement, donations, and distribution to ensure equitable access to vaccines where they are most needed, along with other public health tools, to prevent infections, stop transmission, and save lives.”
Global Impact
Since the start of the global MPOX outbreak in 2022, some 120 countries have confirmed over 103,000 cases. The WHO’s prequalification of the MVA-BN vaccine is based on the evaluation of information submitted by the manufacturer, also reviewed by the European Medicines Agency, the regulatory agency of record for this vaccine. The safety and efficacy of the vaccine have been tested in clinical studies and in real-world settings, including in different contexts with the emergence of new strains of the virus.
Vaccine Details
The MVA-BN vaccine can be administered to people aged 18 years and older in two injectable doses four weeks apart. After prior cold storage, the vaccine can be stored at 2-8°C for up to eight weeks. The health agency also recommends using a single dose in outbreak situations with supply constraints and stresses the need to collect more data on the number of doses.
Off-Label Use
Although the MVA-BN vaccine is not currently licensed for use in persons under 18 years of age, it can be used “off-label” in infants, children, and adolescents, and in pregnant and immunocompromised individuals in outbreak contexts where the benefits of vaccination outweigh the potential risks.
Efficacy
To date, data show that a single dose of MVA-BN vaccine administered prior to exposure is estimated to be 76% effective in protecting people against MPOX, and the two-dose regimen is estimated to be 82% effective. Post-exposure vaccination is less effective than pre-exposure vaccination.
Next Steps
The WHO has conducted programmatic and product capacity assessments since the Director-General activated the emergency use listing for MPOX vaccines on 7 August 2024. The health agency is advancing in the procedures for prequalification and inclusion in the list of vaccines for emergency use with the manufacturers of two other MPOX vaccines. Additionally, the WHO has received six expressions of
How does the approval of the first MPOX vaccine by the WHO impact the outbreak in Africa?
Breaking News: WHO Approves First MPOX Vaccine, Aiming to Curb Outbreak in Africa
In a significant breakthrough, the World Health Organization (WHO) has granted approval for the use of the first MPOX vaccine, paving the way for timely access to millions of people at risk in Africa, where the latest outbreak has infected over 20,000 individuals this year alone. The Danish pharmaceutical company, Bavarian Nordic’s MVA-BN vaccine, has already received approval in Europe and the United States for use in adults.
Fighting the MPOX Outbreak
The WHO’s prequalification of the vaccine marks a crucial step in the global fight against the mpox virus. According to WHO Director-General Tedros Adhanom Ghebreyesus, “We now urgently need to scale up procurement, donations, and distribution to ensure equitable access to vaccines where they are most needed, along with other public health tools, to prevent infections, stop transmission, and save lives.”
Since the start of the global mpox outbreak in 2022, a staggering 120 countries have confirmed over 103,000 cases. The urgent need for a viable vaccine has been evident, and the WHO’s approval is a beacon of hope in the quest to control the outbreak.
Vaccine Efficacy and Safety
The MVA-BN vaccine has undergone rigorous clinical trials and real-world testing, demonstrating its safety and efficacy in different contexts, including the emergence of new strains of the virus. The vaccine can be administered to individuals aged 18 years and older in two injectable doses, four weeks apart. After prior cold storage, the vaccine can be stored at 2-8°C for up to eight weeks.
The WHO recommends using a single dose in outbreak situations with supply constraints, emphasizing the need for further data collection on the number of doses. The health agency has reviewed all available evidence and recommends the use of MVA-BN vaccine in the context of an mpox outbreak for people at high risk of exposure.
Off-Label Use and Future Directions
Although the MVA-BN vaccine is not currently licensed for use in persons under 18 years of age, it can be used “off-label” in infants, children, and adolescents, as well as in pregnant and immunocompromised individuals, where the benefits of vaccination outweigh potential risks.
Future research will focus on collecting more data on the number of doses and the vaccine’s effectiveness in different populations. With a single dose of MVA-BN vaccine estimated to be 76% effective in protecting people against mpox, and the two-dose regimen estimated to be 82% effective, the WHO’s approval marks a significant milestone in the fight against this global health threat.
Conclusion
The WHO’s approval of the first MPOX vaccine is a crucial step in controlling the outbreak in Africa and beyond. With timely access to this vaccine, millions of people at risk will be able to benefit from this life-saving intervention. As the global health community continues to work together to combat this outbreak, the WHO’s approval stands as a testament to the power of collaboration and innovation in the pursuit of public health.
Keywords: MPOX vaccine, WHO approval, mpox outbreak, Africa, Bavarian Nordic, MVA-BN vaccine, vaccine efficacy, public health.