antibody are an ingenious patent of our immune system. These small protein molecules can be mass-produced quickly and have a highly specific effect: according to the lock-and-key principle, they dock onto specific surface molecules of pathogens or diseased cells and kill them. The prerequisite for this, however, is that the immune system has previously identified these molecular markers and produced the appropriate antibodies.
Monoclonal Antibodies
This is exactly where many antibody therapies once morest cancer come in: because the immune system often does not recognize the tumor cells and therefore does not produce any or too few antibodies on its own, doctors help. To do this, they first look for antigens on the surface of the cancer cells that distinguish them from normal body cells and are therefore specific to the cancer. For example, some breast cancer tumors overproduce the HER2/neu receptor.
In order to produce suitable antibodies once morest these cancer antigens in the laboratory, mice are first “vaccinated” with the antigen. Your immune system then forms suitable antibody-producing B lymphocytes. These cellular antibody factories are multiplied in cell culture and now produce large amounts of the appropriate immunoglobulins, the so-called monoclonal antibodies. Cancer patients with the right tumor type can then receive these antibodies as an infusion
One of the first monoclonal antibodies once morest cancer approved in Germany is trastuzumab (Herceptin). It is used once morest metastatic breast cancer when the cancer cells carry the HER2/neu receptor on their surface – which is the case in around one in four breast cancer patients. Antibody therapy is usually carried out in combination with classic chemotherapy. Monoclonal antibodies are also used in lymphoma and leukemia.
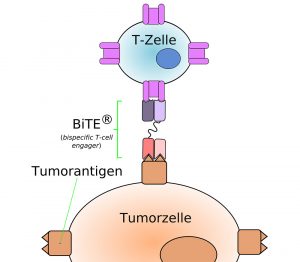
BiTE: Antibody with a double bite
Antibody preparations can incapacitate a cancer cell in different ways. Some trigger apoptosis by docking – the tumor cells die as a result of the cell’s own suicide program. Other antibodies seek help: their free Fc ends are recognized by the immune system’s killer and scavenger cells, which then kill the tumor cell marked in this way.
A new, particularly promising type of such bispecific antibodies are so-called BiTE antibodies (bi-specific T-cell engager). These artificially produced molecules consist of a light chain and a heavy chain of two different monoclonal antibodies. One side of these antibodies can dock onto the receptors of cancer cells. The other side binds to T cells, which then release cytotoxic molecules and kill the cancer cell. The first preparations with such BiTE antibodies are currently being tested in clinical studies, and the drug blinatumomab has been approved in the EU for the treatment of certain lymphomas and leukemias since 2015.
Only active on the tumor
However, antibodies that attract immune cells in this way have a disadvantage: immune cells often react to the appropriate ends of the antibodies before they even reach the tumor. This can cause significant side effects. A team led by Harald Kolmar from TU Darmstadt has a possible solution for this in 2021 presented: “The goal of our work was to find a way to temporarily block the immune stimulation of the antibody and only activate it directly on the tumor,” explains Kolmar.
To do this, the researchers cover the Fc end of the therapeutic antibodies with a protein cap, making the molecules virtually invisible to the immune system. Cancer’s own enzymes only detach this cap at the tumor and enable the defense cells to dock and become active. These antibodies, which can be switched off, have already worked well in cell cultures, and animal experiments are now to follow.
Tumors as antibody factories
You can even convert the cancer cells themselves into antibody factories – the cancer thereby creates defense molecules once morest itself. Such an approach was successfully tested by a team led by Sheena Smith from the University of Zurich in mice with breast cancer in 2021. The basis of the therapy called SHREAD (SHielded, REtargeted Adenovirus) is a viral gene shuttle that contains the genetic blueprint for the appropriate antibody – in this case trastuzumab. Special molecules on the virus surface ensure that the gene shuttle can only penetrate tumor cells, but not normal body cells.
Once in the tumour, the antibody blueprint is built into the cellular DNA and read at the same time. As a result, the cancer cells now produce trastuzumab themselves and thus kill themselves. The big advantage: the antibodies only have a targeted effect in the tumor and reach high concentrations there, but are hardly distributed in the blood and other organs. This reduces side effects. So far, however, this therapy has only been tested in animal experiments, clinical studies in humans are still pending.
December 2, 2022
– Nadja Podbregar
