- André Biernath – @andre_biernath
- BBC News Brazil
2 hours ago
Photo credit, Getty Images
The treatment of certain tumors has undergone a real revolution over the past ten years.
Each year, tens of thousands of physicians from around the world gather in Chicago, USA to learn regarding the latest innovations in cancer diagnosis and treatment.
In 2022, the information presented at the meeting of the American Society of Clinical Oncology made physicians and patients particularly optimistic.
According to experts, the research published this year brings important advances that change the perspective of the fight once morest various types of tumors.
Here’s what the major breakthroughs announced may mean for cancer treatment.
Breast cancer: the drug benefits many more patients
The drug trastuzumab has been used in the treatment of breast cancer for decades.
However, despite the good results, it always had a limit: it might only be prescribed to patients with tumors that expressed a lot of a gene called HER2, which is verified by an examination.
But that has now changed: One of the big news from Congress was the results of the study on the drug trastuzumab deruxtecan.
“We are witnessing the arrival of a revolutionary medicine,” says oncologist Romualdo Barroso, research coordinator at the Sírio-Libanês Hospital in Brasilia.
“After many years without major news, we have a new treatment option that increases survival (longer life) for patients.”
According to Barroso, the new remedy works like a Trojan horse (meaning it looks like one thing, but works like another).
Trastuzumab is a monoclonal antibody, a type of medicine that can be used to both prevent and treat disease. In the case of breast cancer, it binds to receptors on the surface of cancer cells.
This has two main effects. The first is to “catch the attention” of the immune system, which begins to see the cancer as a threat and initiates a series of actions to combat it.
The second is to allow deruxtecan (the second part of the drug) to “invade” diseased cells. It is a powerful chemotherapy drug that destroys the tumor from the inside.
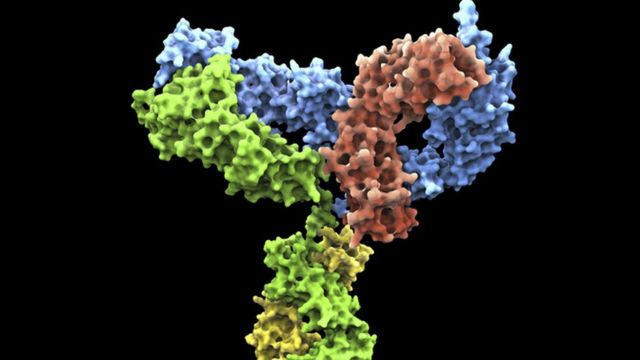
Photo credit, Getty Images
Monoclonal antibodies are made in the laboratory to “stick” to the surface of tumors and trigger a counterattack.
But the novelty goes beyond how it works: the new drug works well even in patients with tumors that express less of the HER2 gene.
This means in practice that more people can benefit from this remedy. Nearly seven out of ten patients, Barroso estimates.
The drug, which is given into a vein every 21 days, is still awaiting approval from regulators for use in hospitals.
At first it can be used as a second line of treatment, that is, when the first options have failed and the disease has spread to other parts of the body (a process called metastasis).
According to Barroso, it is likely that over time this will also become an option for early-stage tumours.
Rectal cancer: drug with surprising results (even for doctors)
Imagine a drug that manages to make a disease disappear in all the patients in the study conducted to analyze whether it works or not.
Naturally, such a positive result attracts the attention of those who are not specialists in the matter.
“But even for us doctors, it’s very surprising,” says oncologist Rachel Riechelmann, director of the clinical oncology department at the AC Camargo Cancer Center in São Paulo.
This is exactly what happened in a trial of dostarlimab to treat cancer of the rectum (the last part of the intestine). It is already used for other tumours, such as those affecting the endometrium (tissue that covers the uterus).
Dostarlimab belongs to the class of immunotherapies, which stimulate the immune system to attack the tumour.
The study involved 12 patients who were followed for six months. In the end, all of them no longer had any evidence of a tumor in the body.
This saved them from having to switch to more aggressive treatments, such as surgery, radiation therapy or chemotherapy.
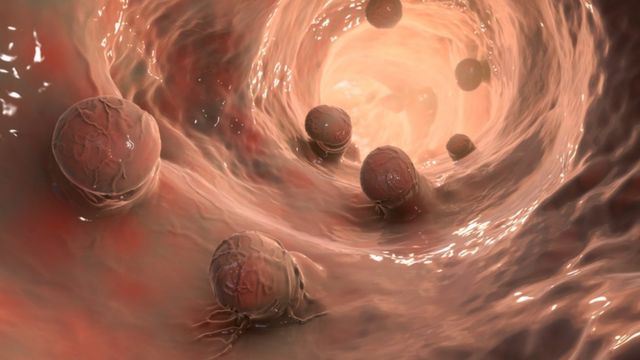
Photo credit, Getty Images
Tumors that appear in the rectum have been treated with surgery, radiation therapy, or chemotherapy.
Although the result is impressive, some considerations should be made.
The first has to do with tracking time. “The six months of evaluation is a short period. It is possible that the disease will reappear a few years later”, analyzes Riechelmann.
Second, dostarlimab only works in a limited group of patients who have tumors with a characteristic described as “microsatellite instability.” It is estimated that regarding 1% of rectal cancer cases meet this criterion.
Although the drug is not approved for the new use, research continues even to find out how long patients actually live without this tumor.
“But the first results were so good that it no longer makes sense to compare this immunotherapy with what was used before, such as chemo and radiation therapy,” says Riechelmann.
“It’s a treatment that has proven to be better and less toxic,” he concludes.
Colorectal cancer: the examination avoids unnecessary chemotherapy
Generally, international oncology congresses bring advances related to new tools, diagnostic methods and, of course, drugs.
However, this year, work on colorectal cancer (which affects parts of the large intestine) was put forward precisely for having followed the opposite path: reducing the number of interventions to which the patient must submit.
A group of researchers from Australian institutions has evaluated a test that detects bits of tumor DNA that appear in the bloodstream. The method is known as “liquid biopsy”.
But what does this have to do with colorectal cancer? Patients diagnosed with this disease usually undergo surgery to remove the affected part of the intestine.
After healing, however, the doctor always wonders if there is even a microscopic part of the tumor left in the patient’s body. If it remains, the disease can regrow and even spread throughout the body.
Just in case, many people undergo chemotherapy following surgery to remove any tumor cells that got in the way.
This reduces the risk of relapses, but subjects patients to heavy therapy, which can have side effects.
This is where the new test comes in: by detecting the pieces of tumor DNA, it determines who really needs the second round of treatment.
“If the result of the liquid biopsy is positive, he goes to chemo. If he is negative, he does not need it”, summarizes oncologist Rodrigo Dienstmann, medical director of Oncoclínicas Precision Medicine, in São Paulo.
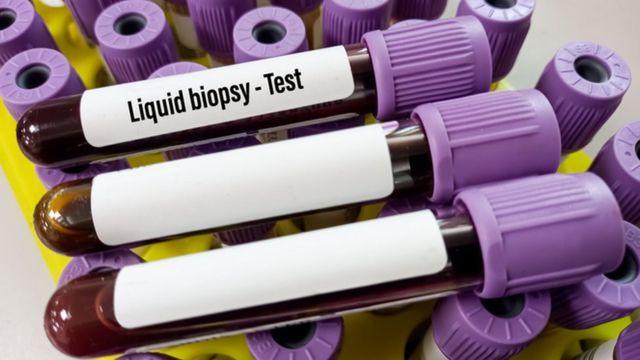
Photo credit, Getty Images
The liquid biopsy will help guide future cancer treatments, doctors say.
In the study that validated the technique, 455 volunteers were divided into two groups. The first 302 had the liquid biopsy right following the surgery. With the remaining 153, the doctor decided whether or not to go for chemo.
“Among those who had a liquid biopsy, 15% went to chemo followingwards. Among the others, 28%,” informs Dienstmann.
“That is to say, it was possible to reduce the application of chemotherapy by half and to obtain the same result in patient survival,” he compares.
“Liquid biopsy has revolutionary potential”, analyzes the doctor.
Pancreatic cancer: hope for a successful treatment
Pancreatic adenocarcinoma may top the list of tumors with the worst prognosis.
“This cancer has a very high mortality. About 90% of patients do not survive for five years, even when diagnosed early,” explains Dr. Paulo Hoff, President of Oncologia D’Or.
Over the last ten years, the evolution of the treatment of this disease has been limited to the arrival of new chemotherapies — the progress linked to more modern and less aggressive drugs, such as immunotherapies and monoclonal antibodies, has not not benefited in this case has this disease which affects the pancreas.
But a new possibility has opened up: at this year’s American Oncology Congress, the first tests using a method called CAR-T Cells once morest this type of cancer were presented.
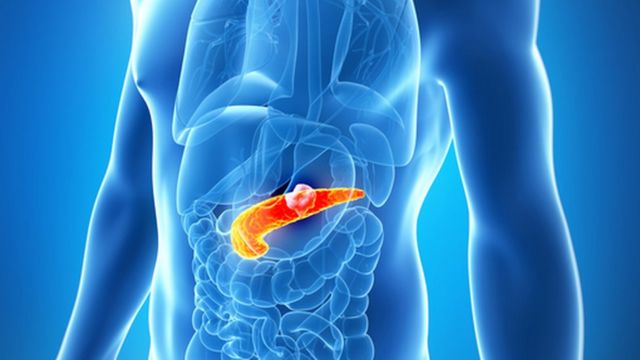
Photo credit, Getty Images
Pancreatic cancer is often aggressive and has few more modern treatment options.
The therapeutic resource, already approved once morest certain blood tumors (such as lymphomas, leukemias and multiple myeloma), consists of extracting the patient’s own immune cells, modifying them in the laboratory and reintroducing them into the body, so that recognize and attack the tumour.
According to what was presented at the congress, CAR-T cells were tested in a patient with pancreatic cancer in the United States. The first results were positive.
“Although the use of this therapy once morest pancreatic adenocarcinoma is extremely interesting, it is not something that will be available in our clinics tomorrow,” reflects Hoff, professor of clinical oncology at the University of São Paulo.
“There is still a long way to go, but at least now we have hope that we can be on the right track.”

