By studying the behavior of water at very high pressure, a team from the University of Nevada in Las Vegas (UNLV) discovered a new form of ice, denoted Ice-VIIt, whose arrangement of atoms had never before been observed. The researchers think that this form of solid water might be particularly abundant in the crust and the upper mantle of certain exoplanets, which might therefore present a greater potential for habitability.
In liquid water, although the water molecules are mobile, each is surrounded at all times by four other water molecules (the four oxygen atoms forming a tetrahedron). Below 0°C, at atmospheric pressure, liquid water changes to a solid state: this “ordinary” form of ice then adopts a crystalline structure of the hexagonal type, in which the hydrogen atoms are disordered; this variety of ice cream is called ice Ih. It is the ice that forms in our freezers or falls as hail or snowflakes. Its density is less than that of water.
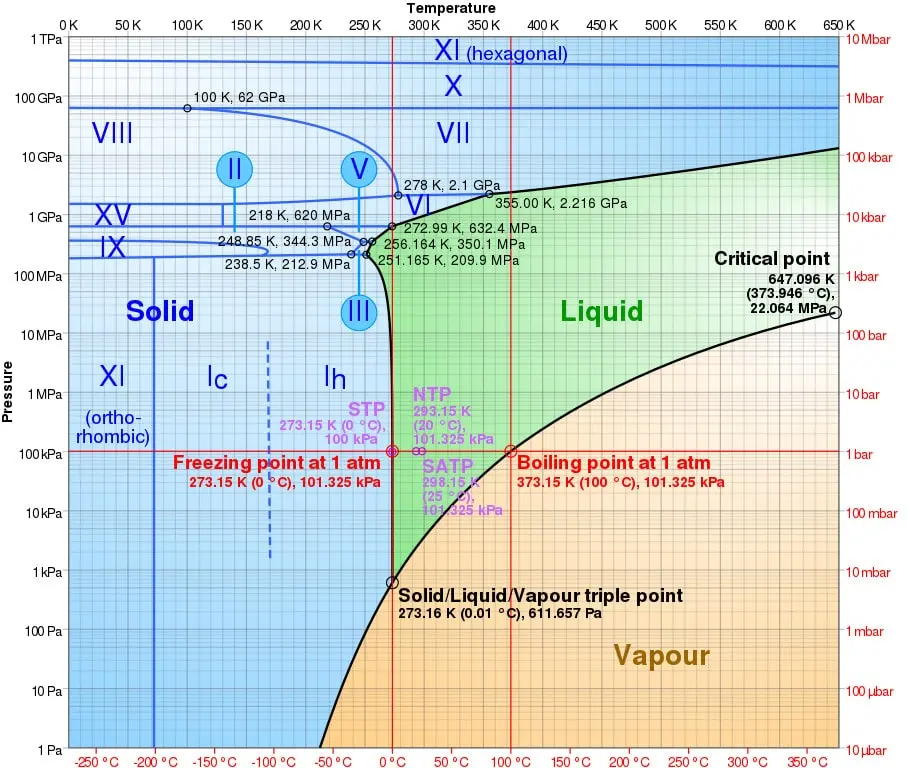
But depending on the temperature and pressure conditions, water can actually form several other forms of solids (see diagram below), more or less stable. Scientists have already identified more than twenty forms of ice. Under high pressure in particular, there are more than ten crystalline forms (denoted ice or ice II, III, IV, etc.). Unlike the standard ice that we know, these ices are characterized by a higher density than the corresponding liquid water – which means that ice cubes made of this particular ice would sink to the bottom of a glass of water.
A transition between two known ice phases
Using a new method designed to measure the properties of water at high pressure, researchers at UNLV’s Nevada Extreme Conditions Lab observed a novel water ice transition, located between ice phases VII and ice X already well known. To achieve this novel shape, the team, led by Zachary Grande, placed a sample of water in a diamond anvil cell — a device that allows material to be subjected to extreme pressures and temperatures, the sample being able to be heated by an infrared laser up to 4000 K.
The sample was compressed between the two diamonds, until it froze into several mixed ice crystals. They then temporarily melted this ice by laser; it then quickly resolidified in the form of tiny crystals, like a powder. They repeated the operation, gradually increasing the pressure, periodically melting the ice with a laser and observing how it recrystallized — the oxygen and hydrogen atoms adopting a different arrangement each time.
 100vw, 800px”/>
</picture>
</noscript><figcaption id=) Semi-logarithmic pressure-temperature phase diagram of water. The Roman numerals correspond to the different phases of ice. © Cmglee – CC BY-SA 3.0
Semi-logarithmic pressure-temperature phase diagram of water. The Roman numerals correspond to the different phases of ice. © Cmglee – CC BY-SA 3.0This is how they observed the ice change from a known cubic phase (ice VII), to a structure of tetragonal symmetry never seen before, denoted ice VIIt, before stabilizing in another known phase (ice X). The transition to ice VIIt was observed at 5.1 ± 0.5 GPa, report the researchers in Physical Review B.
Namely, Ice VII was first synthesized in 1937; it was observed in its natural state in 2017, in the form of inclusions in diamonds extracted from a mine in Botswana – these inclusions formed in the Earth’s mantle, in the state of liquid water, which s is crystallized as it rises to the surface. It is a simple cubic phase, in which the positions of the hydrogen atoms are disordered. Ice X, also observed at high pressure, adopts a centered cubic structure.
Ice that might be common on some exoplanets
In Ice Phase VIItice VII’s cubic lattice is stretched along one of its vectors so that the structure expands into a rectangular arrangement, and then it eventually settles into the fully ordered symmetric cubic ice arrangement X.
Along the way, the team also discovered that the transition to phase X occurred at much lower pressures (nearly three times lower!) than expected: the hydrogen bond symmetrization transition produced at a pressure of 30.9 ± 3 GPa, they specify.
However, this particular transition has been the subject of debate for several decades among physicists – previous research work placed this transition pressure between 40 and 120 GPa. ” Zach’s work demonstrated that this transformation to an ionic state occurs at much, much lower pressures than previously thought. It’s the missing piece, and the most accurate measurements ever made on the water in these conditions. », souligne Ashkan Salamatphysicist from UNLV.
Given the conditions necessary for its formation, ice cannot be observed VIIt on the surface of the Earth. By contrast, the researchers believe it might be relatively common in Earth’s mantle, as well as large moons and water-rich planets outside our solar system. This discovery thus calls into question the habitability potential of certain exoplanets.
