[의학신문·일간보사=이승덕 기자]The target of national support for ‘human papillomavirus infection (HPV) vaccination’ will be extended to female adolescents and low-income women.
The Korea Centers for Disease Control and Prevention (CEO Jeong Eun-kyung) announced that it plans to expand the number of recipients of human papillomavirus infection (HPV vaccination) support, which is currently being implemented as part of the Healthy Women’s First Step Clinic project, from the 14th.
Currently, the target of national support for HPV vaccination is 12 years old, and it is applied to women and adolescents born from January 1, 2009 to December 31, 2010.
From March 14, 13-17 years old (January 1, 2004 – December 31, 2008) and 290,000 female adolescents and 18-26 years old (January 1, 1995 – December 31, 2003) ) Expand the target of national support to 100,000 low-income women.
Because the HPV vaccine has a wide range of ages for approval by the Ministry of Food and Drug Safety, there has been a steady request to extend the target age from 12 years old, which is the existing support age, and it was accepted and expanded. The approval age of the Ministry of Food and Drug Safety is 9 to 26 years for the HPV quadrivalent vaccine and 9 to 25 years for the HPV bivalent vaccine.
In addition, since the HPV vaccine is more expensive than other vaccines (the average cost of inoculation for HPV 4 is 167,551 won and HPV 9 is 204,497 won), the support has been expanded to women from low-income families so that they can access the vaccine.
In the case of low-income women, vaccination support is available only to those who are eligible for guaranteed benefits on the day of vaccination.
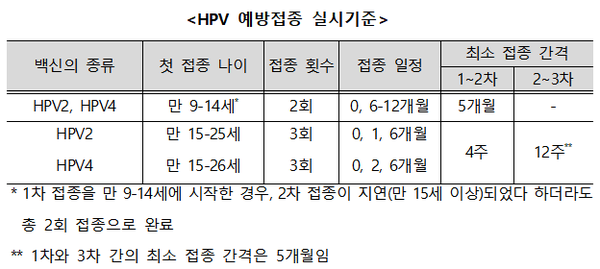
Since the number of times and intervals between vaccinations are different depending on the age of vaccination and the type of vaccine, HPV vaccination should be performed according to the age of the individual following a doctor’s examination before vaccination.
HPV vaccination can effectively prevent infection with HPV, which can progress to cervical cancer, oropharyngeal cancer, and anus-genital cancer. It is very important to vaccinate before.
The Global Advisory Committee on Vaccine Safety (GACVS) regularly evaluates the safety of HPV vaccines and rated HPV vaccines as very safe.
After inoculation, pain, swelling, and redness may occur at the inoculation site, but most of these local adverse reactions disappear spontaneously within a few days. It can be prevented by taking measures such as sitting or lying down for 20 to 30 minutes followingward.
Since the HPV vaccine was introduced as a national vaccination in Korea in June 2016, 176 cases (0.0082%) of adverse reactions have been reported following regarding 2.14 million inoculations. There were 68 cases of dizziness before syncope.
Other adverse reactions were reported following inoculation, including allergic and skin adverse reactions, local reactions, fever, and headache.
Commissioner Jeong Eun-kyung said, “In 2019, 3,273 people were diagnosed with cervical cancer and 898 people died from the same disease (Statistics of the cause of death, Statistics Korea). Please get vaccinated once morest HPV so that they can improve their health.”

